The hyperactivating Rac1P29S mutation drives drug tolerance and metastasis in melanoma
A lab team orchestrated by Ashwathi (Abbee) Mohan discovered that the molecular infrastructure that allows a cell to control its shape and migrate can also be engaged in cancer cells to increase signals that promote growth. The way these processes are linked is through branched actin filament networks. These networks can extrude very flat sheets called lamellipodia. In the context of cell migration, these lamellipodia are at the front of the cell, setting the direction the rest of the cell will follow. Mohan et al. now show that in melanoma, these lamellipodia trap tumor suppressor molecules, whose job is to limit cell growth; and, in addition, lamellipodia trap a whole slew of other molecules of which some have the ability to inactive these tumor suppressors. Thus, as a cell protrudes it inactivates some of the processes that keep cell growth under control.
In normal cells, these protrusions are small enough to not significantly affect cell growth. Combining gene editing, molecular cell biology, advanced live cell imaging, and computer vision Mohan and her colleagues found that an activating mutation in the molecule Rac1, which drives lamellipodia formation, can lead to massively extended and denser actin filaments. This mutation was isolated in melanoma in 2012 by two labs independently at Yale and MD Anderson Cancer Center. About 10% of melanoma patients carry the mutation. In 2014, the MD Anderson group showed that this mutation is among the culprits that can turn melanoma cells resistant to standard of care chemotherapy of this cancer. The paper published in Developmental Cell now unravels the process underlying this drug resistance. And it even shows that the lack of growth suppression related to the Rac1 mutation leads to massively larger metastatic burden caused by melanoma in mice.
In an interview with Deborah Wormser form UTSW’s Newsroom, Abbee says, “It’s almost like a super power that some cancer cells have that lets them grow even under difficult conditions such as under drug treatment or in foreign tissue environments at metastatic sites. Under stress, when most melanoma cells stop growing, certain cells are able to turn on their lamellipodia and re-purpose them to sustain growth signals. This ability to grow under stress gives these cells the ability to resist drugs but also to grow more aggressively after spreading to different parts of the body.”
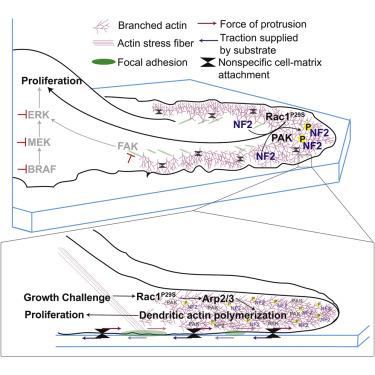 Hyperactivation of Rac1 via the P29S mutation (Rac1P29S) in melanoma hijacks branched actin network assembly to coordinate proliferative cues that facilitate metastasis and drug resistance.
Hyperactivation of Rac1 via the P29S mutation (Rac1P29S) in melanoma hijacks branched actin network assembly to coordinate proliferative cues that facilitate metastasis and drug resistance.