Akbay Lab studies genetic and molecular events that lead to lung-tumor initiation and immune evasion.
Tumor cells and immune cells share limited nutrients in the environment. Using high throughput techniques and functional analysis we study the contribution of tumor cell metabolism to immune evasion.
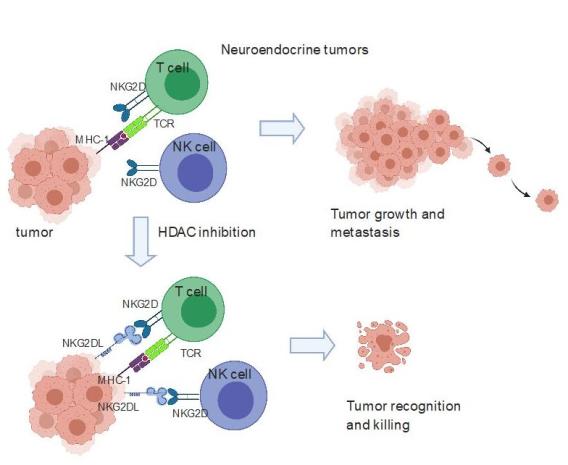
Small cell lung cancer (SCLC) is one of the most aggressive cancer types with exceptionally poor survival rates irrespective of stage. There has been minimal progress in the treatment of SCLC over the last 30 years. Recently PD-1 blocking antibody was approved to be used in combination with chemotherapy in SCLCs. However, for unknown reasons PD-1 blockade is effective in only a very small subset of SCLCs. We observed that SCLC cell lines and patient tumor tissue lack protein expression of ligands for the most potent NK cell activating receptor NKG2D and thus not visible to NK and T cells. We showed that epigenetic silencing contributes to the lack of NKG2DL expression in the neuroendocrine subtype of SCLC. Restoration of NKG2DL by treatment with an HDAC inhibitor upregulated NKG2DLs in lung cancer cell lines. Additionally, this treatment sensitized a syngeneic SCLC model to NK and T cell killing in vivo. Similar observations were made in another neuroendocrine tumor, neuroblastoma. We discovered a lineage-dependent mechanism of immune escape for SCLC and neuroblastoma and a pharmacological approach to activating anti-tumor innate immunity against this designated “recalcitrant cancer” (Zhu et al Can Res). Our current projects are focused on harnessing this vulnerability of SCLCs to target them to immune cell killing.
Amino acid metabolism is recognized as a critical immune regulatory control point, which tempers T cell response. Tryptophan degradation limits innate and adaptive immune responses by depleting the tumor local microenvironment of tryptophan and by promoting the accumulation of kynurenine resulting from the catabolism of tryptophan. Similar to tryptophan metabolism, arginine degradation contributes to T cell suppression. While most cells can synthesize arginine from citrulline, T cells are dependent on extracellular arginine. Arginases and NOS are the main enzymes regulating extracellular arginine levels in the tissues. Though the exact role of activation of arginase across the different genomic subtypes of lung cancer is not fully understood, we showed that targeting arginase is an effective therapeutic strategy in Kras mutant lung cancers (Miret et al, JITC).
Alterations in other metabolites such as Adenosine have also been implicated in immune suppression. We showed that EFGR mutations activate the Adenosine pathway and suppress anti-tumor immunity through the accumulation of adenosine in the tumor tissue and increased uptake by the T cells. Our current projects are focused on understanding the role of these altered metabolic pathways in immune resistance in genomically classified tumors using transgenic, syngeneic, and humanized cancer models.
We discovered a novel splice isoform of major immune checkpoint protein PD-L1. This isoform retains part of an intron and lacks the transmembrane domain. Unlike the full-length protein does not localize to the membrane but secreted into the media or circulation. This soluble isoform still retains the ability to bind to the PD1 receptor and is able to suppress T cell activation.

Meet the Principal Investigator

Esra Akbay, Ph.D.
Associate Professor, Department of Pathology
Dr. Akbay earned her Ph.D. from UT Southwestern Medical Center and received her postdoctoral training in translational lung cancer research from the Dana-Farber/Harvard Cancer Center.
Lab Members
Qing Deng, Ph.D.
Post-Doctoral Fellow
Ph.D.: Shanghai Jiao Tong University

Ryan Kowash, B.S.
Ph.D. Student
Undergraduate: Dickinson College

William Huang
Ph.D. Student
Undergraduate: Chungshan Medical University, Taiwan

Ateş Kutay Tenekeci
MD/Ph.D. Student

Former Members
Daniella Rin Yang, B.S.
Research Technician II
Undergraduate: Stony Brook University
Current position: MD/PhD student at Oregon Health and Science University

Buse Eglenen Polat, M.D.
Post-Doctoral Researcher
Medical degree: Koç University Medical School, Istanbul
Current position: Hematology and Oncology Fellow at Montefiore Einstein Medical Center

Mingrui Zhu, B.S.
Ph.D. Student
Undergraduate: Wuhan University
Current Position: Postdoctoral Fellow at St. Jude Children's Hospital

Mathew Bender, B.S.
Research Technician II - Lab Manager
Undergraduate: Trinity University
Current position: Medical Student, Texas Tech University

Featured Publications
Tumor intrinsic and extrinsic functions of CD73 and the adenosine pathway in lung cancer.
Kowash RR, Akbay EA, 2023 Front Immunol 14 1130358Evasion of Innate Immunity Contributes to Small Cell Lung Cancer Progression and Metastasis.
Zhu M, Huang Y, Bender ME, Girard L, Kollipara R, Eglenen-Polat B, Naito Y, Savage TK, Huffman KE, Koyama S, Kumanogoh A, Minna JD, Johnson JE, Akbay EA, 2021 Jan Cancer ResIdentification and characterization of an alternative cancer-derived PD-L1 splice variant.
Hassounah NB, Malladi VS, Huang Y, Freeman SS, Beauchamp EM, Koyama S, Souders N, Martin S, Dranoff G, Wong KK, Pedamallu CS, Hammerman PS, Akbay EA 2018 Dec Cancer Immunol. Immunother.Polymerase-mediated ultramutagenesis in mice produces diverse cancers with high mutational load.
Li HD, Cuevas I, Zhang M, Lu C, Alam MM, Fu YX, You MJ, Akbay EA, Zhang H, Castrillon DH 2018 Aug J. Clin. Invest. 9 128 4179-4191Autochthonous murine models for the study of smoker and never-smoker associated lung cancers.
Akbay EA, Kim J 2018 Aug Transl Lung Cancer Res 4 7 464-486D-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice.
Akbay EA, Moslehi J, Christensen CL, Saha S, Tchaicha JH, Ramkissoon SH, Stewart KM, Carretero J, Kikuchi E, Zhang H, Cohoon TJ, Murray S, Liu W, Uno K, Fisch S, Jones K, Gurumurthy S, Gliser C, Choe S, Keenan M, Son J, Stanley I, Losman JA, Padera R, Bronson RT, Asara JM, Abdel-Wahab O, Amrein PC, Fathi AT, Danial NN, Kimmelman AC, Kung AL, Ligon KL, Yen KE, Kaelin WG, Bardeesy N, Wong KK 2014 Mar Genes Dev. 5 28 479-90Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors.
Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, Fecci PE, Butaney M, Reibel JB, Soucheray M, Cohoon TJ, Janne PA, Meyerson M, Hayes DN, Shapiro GI, Shimamura T, Sholl LM, Rodig SJ, Freeman GJ, Hammerman PS, Dranoff G, Wong KK 2013 Dec Cancer Discov 12 3 1355-63Differential roles of telomere attrition in type I and II endometrial carcinogenesis.
Akbay EA, Contreras CM, Perera SA, Sullivan JP, Broaddus RR, Schorge JO, Ashfaq R, Saboorian H, Wong KK, Castrillon DH 2008 Aug Am. J. Pathol. 2 173 536-44Glucocorticoid mediated inhibition of LKB1 mutant non-small cell lung cancers.
Huffman KE, Li LS, Carstens R, Park H, Girard L, Avila K, Wei S, Kollipara R, Timmons B, Sudderth J, Bendris N, Kim J, Villalobos P, Fujimoto J, Schmid S, Deberardinis RJ, Wistuba I, Heymach J, Kittler R, Akbay EA, Posner B, Wang Y, Lam S, Kliewer SA, Mangelsdorf DJ, Minna JD, 2023 Front Oncol 13 1025443Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape.
Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, Kumagai S, Tada Y, Togashi Y, Koyama S, Akbay EA, Karasaki T, Kataoka K, Funaki S, Shintani Y, Nagatomo I, Kida H, Ishii G, Miyoshi T, Aokage K, Kakimi K, Ogawa S, Okumura M, Eto M, Kumanogoh A, Tsuboi M, Nishikawa H, 2021 Nov Sci Immunol 65 6 eabc6424Dysfunctional telomeres trigger cellular senescence mediated by cyclic GMP-AMP synthase.
Abdisalaam S, Bhattacharya S, Mukherjee S, Sinha D, Srinivasan K, Zhu M, Akbay EA, Sadek HA, Shay JW, Aroumougame A, 2020 Jun J. Biol. Chem.Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients.
Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T, Hirata H, Yano Y, Yokota S, Kinehara Y, Naito Y, Otsuka T, Kanazu M, Kuroyama M, Hamaguchi M, Koba T, Futami Y, Ishijima M, Suga Y, Akazawa Y, Machiyama H, Iwahori K, Takamatsu H, Nagatomo I, Takeda Y, Kida H, Akbay EA, Hammerman PS, Wong KK, Dranoff G, Mori M, Kijima T, Kumanogoh A 2018 Oct JCI Insight 19 3Assessing Therapeutic Efficacy of MEK Inhibition in a KRAS G12C-Driven Mouse Model of Lung Cancer.
Li S, Liu S, Deng J, Akbay EA, Hai J, Ambrogio C, Zhang L, Zhou F, Jenkins RW, Adeegbe DO, Gao P, Wang X, Paweletz CP, Herter-Sprie GS, Chen T, Gutierrez Quiceno L, Zhang Y, Merlino AA, Quinn MM, Zeng Y, Yu X, Liu Y, Fan L, Aguirre AJ, Barbie DA, Yi X, Wong KK 2018 Jun Clin. Cancer Res.JAK2/IDH-mutant-driven myeloproliferative neoplasm is sensitive to combined targeted inhibition.
McKenney AS, Lau AN, Somasundara AVH, Spitzer B, Intlekofer AM, Ahn J, Shank K, Rapaport FT, Patel MA, Papalexi E, Shih AH, Chiu A, Freinkman E, Akbay EA, Steadman M, Nagaraja R, Yen K, Teruya-Feldstein J, Wong KK, Rampal R, Heiden MGV, Thompson CB, Levine RL 2018 Jan J. Clin. Invest.Co-clinical quantitative tumor volume imaging in ALK-rearranged NSCLC treated with crizotinib.
Nishino M, Sacher AG, Gandhi L, Chen Z, Akbay E, Fedorov A, Westin CF, Hatabu H, Johnson BE, Hammerman P, Wong KK 2017 Mar Eur J Radiol 88 15-20Lkb1 inactivation drives lung cancer lineage switching governed by Polycomb Repressive Complex 2.
Zhang H, Fillmore Brainson C, Koyama S, Redig AJ, Chen T, Li S, Gupta M, Garcia-de-Alba C, Paschini M, Herter-Sprie GS, Lu G, Zhang X, Marsh BP, Tuminello SJ, Xu C, Chen Z, Wang X, Akbay EA, Zheng M, Palakurthi S, Sholl LM, Rustgi AK, Kwiatkowski DJ, Diehl JA, Bass AJ, Sharpless NE, Dranoff G, Hammerman PS, Ji H, Bardeesy N, Saur D, Watanabe H, Kim CF, Wong KK 2017 Apr Nat Commun 8 14922Development of Selective Covalent Janus Kinase 3 Inhibitors.
Tan L, Akahane K, McNally R, Reyskens KM, Ficarro SB, Liu S, Herter-Sprie GS, Koyama S, Pattison MJ, Labella K, Johannessen L, Akbay EA, Wong KK, Frank DA, Marto JA, Look TA, Arthur JS, Eck MJ, Gray NS 2015 Aug J. Med. Chem. 16 58 6589-606Mutant IDH inhibits HNF-4a to block hepatocyte differentiation and promote biliary cancer.
Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, Miltiadous O, Walesky C, Deshpande V, Zhu AX, Hezel AF, Yen KE, Straley KS, Travins J, Popovici-Muller J, Gliser C, Ferrone CR, Apte U, Llovet JM, Wong KK, Ramaswamy S, Bardeesy N 2014 Sep Nature 7516 513 110-4Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression.
Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, Reibel JB, Walton Z, Ji H, Watanabe H, Jänne PA, Castrillon DH, Rustgi AK, Bass AJ, Freeman GJ, Padera RF, Dranoff G, Hammerman PS, Kim CF, Wong KK 2014 May Cancer Cell 5 25 590-604Image-guided radiotherapy platform using single nodule conditional lung cancer mouse models.
Herter-Sprie GS, Korideck H, Christensen CL, Herter JM, Rhee K, Berbeco RI, Bennett DG, Akbay EA, Kozono D, Mak RH, Mike Makrigiorgos G, Kimmelman AC, Wong KK 2014 Dec Nat Commun 5 5870Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor.
Christensen CL, Kwiatkowski N, Abraham BJ, Carretero J, Al-Shahrour F, Zhang T, Chipumuro E, Herter-Sprie GS, Akbay EA, Altabef A, Zhang J, Shimamura T, Capelletti M, Reibel JB, Cavanaugh JD, Gao P, Liu Y, Michaelsen SR, Poulsen HS, Aref AR, Barbie DA, Bradner JE, George RE, Gray NS, Young RA, Wong KK 2014 Dec Cancer Cell 6 26 909-22Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer.
Shimamura T, Chen Z, Soucheray M, Carretero J, Kikuchi E, Tchaicha JH, Gao Y, Cheng KA, Cohoon TJ, Qi J, Akbay E, Kimmelman AC, Kung AL, Bradner JE, Wong KK 2013 Nov Clin. Cancer Res. 22 19 6183-92Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas.
Contreras CM, Gurumurthy S, Haynie JM, Shirley LJ, Akbay EA, Wingo SN, Schorge JO, Broaddus RR, Wong KK, Bardeesy N, Castrillon DH 2008 Feb Cancer Res. 3 68 759-66Genomewide discovery and classification of candidate ovarian fertility genes in the mouse.
Gallardo TD, John GB, Shirley L, Contreras CM, Akbay EA, Haynie JM, Ward SE, Shidler MJ, Castrillon DH 2007 Sep Genetics 1 177 179-94Lab Fun
Contact the Akbay Lab
Phone: 214-648-4159
Email
Mailing Address
UT Southwestern Medical Center
5323 Harry Hines Blvd.
Dallas, Texas 75390-9072
Join Our Lab
Be part of the impact we're making on cancer research.
A postdoctoral position focused on studying the lung tumor microenvironment and immunotherapy in lung cancer is available in our laboratory. Specific emphasis will be placed on understanding the role of oncogenes and tumor suppressors in evading anti-tumor immunity. This work will incorporate the use of cell culture, animal models, and clinical specimens.
Candidates must hold Ph.D. degree. A cancer research background and a track record of leading projects to publication are preferred. Candidates who have experience with flow cytometry, molecular biology, mouse models, and cell culture techniques are encouraged to apply. The postdoctoral fellow will be expected to direct independent projects in a multi-disciplinary setting and collaborate with the other members of the laboratory.
Interested individuals should send a CV, a statement of interest, and a list of three references to Dr. Esra Akbay.
We are accepting graduate students! Students already enrolled in graduate school can email Dr. Akbay for scheduling rotations.
Prospective students need to apply to the Ph.D. or combined M.D./Ph.D. Programs in UT Southwestern Graduate School, then contact us about rotations.













