
Figure 1: In vivo QBLT-guided conformal irradiation (a1-a3) show a case of a 7-field non-coplanar plan; the contrast-labelled GBM is delineated in blue contour (gross target volume, GTV, used as a ground truth to validate the QBLT reconstructed volume). The planning target volume (PTVQBLT, cyan) is generated with 0.5 mm expansion from the BLT reconstructed GBM volume (GTVQBLT, heat map). The corresponding dose distributions are show in Fig. b1-b3 with 5Gy as the prescribed dose to cover the PTVQBLT.

Figure 2: (a1-c1) QBLT volume-guided vs (a2-c2) CBCT-guided single field irradiation. (a1-a2) are the bioluminescence images from the GBM grown before irradiation. (b1) QBLT-guided conformal RT (3 radiation fields, purple lines) based on BLT-reconstructed GBM volume (heat map) covered by the prescribed dose 10 Gy (red line). Blue contour shows the contrast-labelled GBM. (b2) is single-field RT; 10 Gy was prescribed at 3 mm depth from the surgical opening, where GBM cells were implanted. Orange arrow pointed out the area of tumor (blue line) under dosage from the prescribed dose (red line). Bioluminescence images 3 days after the BLT-guided and single field RT are shown in (c1) and (c2), respectively.
 Figure 3: (a) Computer-aided design of the mobile FT system; (b) shows the interior setting of the system, and (c) is the system with black cloths covering the imaging chamber to prevent ambient light leaking into the imaging chamber.
Figure 3: (a) Computer-aided design of the mobile FT system; (b) shows the interior setting of the system, and (c) is the system with black cloths covering the imaging chamber to prevent ambient light leaking into the imaging chamber. 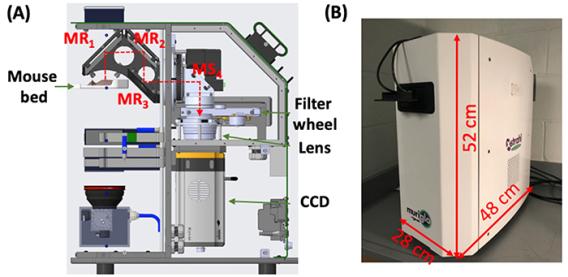 Figure 4: (A) Cross-section of the commercial system (MuriGlo); the optical signal emitted from the animal is reflected through the 3-mirror system (mirrors MR1-MR2-MR3) and the fixed mirror (MS4) to filter, lens and CCD. (B) Shows the actual layout of the system.
Figure 4: (A) Cross-section of the commercial system (MuriGlo); the optical signal emitted from the animal is reflected through the 3-mirror system (mirrors MR1-MR2-MR3) and the fixed mirror (MS4) to filter, lens and CCD. (B) Shows the actual layout of the system.  Figure 5: Workflow layout of the universal QBLT system. After optical imaging, animal is transported on mouse bed to irradiator, and the bed is docked into bed adapter installed in irradiators. A different adapter allows to include MRI acquisition with minimal perturbation of mouse positioning.
Figure 5: Workflow layout of the universal QBLT system. After optical imaging, animal is transported on mouse bed to irradiator, and the bed is docked into bed adapter installed in irradiators. A different adapter allows to include MRI acquisition with minimal perturbation of mouse positioning. Multimodal Quantitative Imaging-Guided System for Pre-Clinical Radiation Research
Unmet needs in current pre-clinical radiation therapy (RT) research include the following: 1) knowing the target’s shape is fundamental for guiding radiation because over-irradiating normal tissue or underdosing tumors can lead to undesired experimental uncertainties; and 2) functional imaging-guided small animal irradiators are not readily available to perform biology-guided RT methods to support translational studies. Moreover, functional imaging is an important technology in pre-clinical research, thus allowing treatment response to be robustly quantified to strengthen scientific findings.
Cone-beam CT/CBCT-guided small animal irradiator has been widely used to test radiobiological hypotheses to closely mimic human treatment. However, it is less adept at localizing soft tissue targets that grow in a low-image contrast environment; also, it does not provide functional information. In contrast, bioluminescence and fluorescence imaging offer strong image contrast and functional information, so they are attractive solutions for soft-tissue imaging guidance. However, 2D optical imaging commonly used on animal surfaces is inadequate to guide irradiation because optical transportation from an internal optical target is highly susceptible to the effects of irregular torso and tissue optical properties.
Recognizing these limitations have led us to integrate 3D bioluminescence tomography (BLT) and fluorescence tomography (FT) with small animal irradiators. In optical tomography, we use a forward model of light propagation through tissue to animal skin surface, in conjunction with an optimization algorithm, to reconstruct the underlying 3D source distribution.
Quantitative Bioluminescence Tomography (QBLT)
Radiation planning in mouse brain based on BLT reconstructed orthotopical glioblastoma is shown in Figure 1a1-3 (heat map). With the knowledge of the BLT-generated tumor shape, we applied conformal radiation to irradiate the target, like in clinical RT. We observed a significant decrease in bioluminescent signal/tumor viability in response to BLT-guided conformal RT (Figure 2). However, in conventional single field irradiation, without proper image-guided system, part of GBM was not covered by the prescribed dose (orange arrow in Figure 2b2). The therapeutic effect is clearly insufficient (Figure 2c2). A proper image-guided system is needed for obtaining adequate experimental conclusions.
Fluorescence Tomography (FT)
Small animal radiation research can be greatly enhanced with biological and functional targeting and assessment capabilities. Fluorescence imaging and tomography (FT) allow in-depth biological and mechanistic interrogation of key information about the tumor and its microenvironment, such as hypoxic burden and metabolic rate, which are known to impact response to RT. FT complements bioluminescence imaging to study tumor and normal tissue response when engineered bioluminescent models are unavailable. Fluorescence imaging is also amenable to clinical translation as a surrogate for PET imaging. Integrated CBCT/BLT/FT capabilities that complement the small animal irradiator would provide a powerful platform for pre-clinical radiation research. UTSW BIRTLab and Xstrahl teams are collaborating through an academic-industrial partnership to develop and commercialize these new modalities. We have been constructing prototype systems to improve testing and design.
A highly flexible mobile BLT/FT system with interchangeable components for various applications is shown in Figure 3. The system consists of a CCD camera and filter wheel for taking spectral images. A rotary stage and 3-mirror assembly are in the optical path to allow images to be taken at viewing angles of 360° around the imaged object/mouse. A universal mouse bed with fiducials was designed to hold the mouse for registration between different imaging modalities. An excitation laser and galvo mirrors were used to position the laser spot onto the mouse to excite fluorescent optical probes. A heater and PID controller were also incorporated in the enclosure to regulate the temperature.
Diffuse Optical Tomography (DOT)
Knowing tissue optical properties ultimately determines BLT/FT reconstruction accuracy. DOT is a non-contrast imaging technique in which light is injected into a tissue on its boundary; the light exiting the tissue can be measured later. By optimizing the algorithm and knowing the input and exiting light, we can then reconstruct selected organ/tissue optical properties at the proper bioluminescence or fluorescence target wavelengths. We anticipate that integrating DOT with our BLT/FT system will improve BLT/FT accuracy in target localization and its quantitative imaging capability for radiation guidance.
Academic-Industrial Partnership
We have translated our know-how to our industrial partner Xstrahl, and our partnership has led to a commercial optical platform MuriGlo for pre-clinical RT study (Figure 4). For imaging with the MuriGlo, individual animals are placed on an anesthesia-controlled mouse bed. The optical signal emitted from the animal is reflected from a rotatable 3-mirror (MR1-3) system to a fixed 45° mirror (MS4) and then through the filter and lens so it can be captured by the CCD camera. The motorized 3-mirror system supports 360° multiple projection imaging.
To broaden the application of the optical system, we also designed the system that can be readily adapted to most commercial irradiators. A new universal mouse bed has been designed to allow the transportation of the anesthetized animal and its accurate re-positioning between the imaging and irradiators. The bed includes 2 parts, mouse bed adapter and transportable mouse bed. The adapter can be placed in Xstrahl small animal radiation research platform (SARRP) and X-RAD SmART system. The transportable bed can carry the animal from MuriGlo, and dock to the bed adapter on both commercial irradiators (Figure 5). A narrower version of the mouse bed can be used in conjunction with MRI machine by using a MRI bed base which can sit in common MRI imaging shell.
References:
- Y. Yang, K. K-H Wang, S. Eslami, I. Iordachita, M. Naser, M. S. Patterson, and J. W. Wong. "Systematic calibration of an integrated x-ray and optical tomography system for preclinical radiation research." Med. Phys., 2015, 42, 1710-1720.
- B. Zhang, K. K-H Wang, J. Yu, S. Eslami, J. Reyes, R. Malek, P. Tran, I. Iordachita, M. S. Patterson, and J. W. Wong. "Bioluminescence tomography-guided radiation therapy for preclinical research." Int. J. Radiat. Oncol. Biol. Phys., 2016, 94, 5, 1144-1153.
- J. Yu, B. Zhang, I. Iordachita, J. Reyes, Z. Lu, M. Brock, M. S. Patterson, J. W. Wong, and K. K-H Wang. “Systematic study of target localization for bioluminescence tomography guided radiation therapy.” Med. Phys., 2016, 43, 2619-2629, DOI: 10.1118/1.4947481.
- B. Zhang, J. W. Wong, I. Iordachita, J. Reyes, K. Nugent, P. Tran, S. W. Tuttle, C. Koumenis, and K. K-H Wang, “Evaluation of on- and offline bioluminescence tomography system for focal irradiation guidance.” Radiat. Res., 2016; 186: 592-601, DOI: 10.1667/RR14423.1
- H. Dehghani, J.A. Guggenheim, S.L. Taylor, X. Xu, and K. K-H Wang. “Quantitative bioluminescence tomography using spectral derivative data.” Biomed Opt Express., 2018; 9: 4163-4174.
- Z. Deng, X. Xu, T. Garzon-Muvdi, A. Luksik, R. Maxwell, J. Yu, I. Iordachita, M. Lim, J. W. Wong and K. K.-H. Wang. “In vivo bioluminescence tomography center of mass-guided conformal irradiation.” Int. J. Radiat. Oncol. Biol. Phys., 2020, 106, 612-620, DOI: 10.1016/j.ijrobp.2019.11.003.
- X. Xu, Z. Deng, H. Dehghani, I. Iordachita, M. Lim, J.W. Wong, and K. K.-H. Wang. "Quantitative bioluminescence tomography-guided conformal irradiation for pre-clinical radiation research." Int. J. Radiat. Oncol. Biol. Phys., 2021, 111, 5, 1310-1321, DOI: 10.1016/j.ijrobp.2021.08.010
- Z. Deng, X. Xu, H. Dehghani, D. M. Sforza, I. Iordachita, M. Lim, J. W. Wong and K. K.-H. Wang. “Quantitative bioluminescence tomography for in vivo volumetric-guided radiotherapy.” In: Ossandon M.R., Baker H., Rasooly A. (eds) Biomedical Engineering Technologies. Methods in Molecular Biology. Vol 2393. Humana, New York, NY. 2022, DOI: 10.1007/978-1-0716-1803-5_38
- A. Bentley, X Xu, Z Deng, JE Rowe, K. K.-H. Wang, and H. Dehghani. “Quantitative molecular bioluminescence tomography.” J. Biomed. Opt., 2022, 27(6), 066004, DOI: 10.1117/1.JBO.27.6.066004
- Z. Deng, X. Xu, I. Iordachita, H. Dehghani, B. Zhang, M. Lim, J. W. Wong, and K. K.-H. Wang, “Mobile bioluminescence tomography-guided system for pre-clinical radiotherapy research.” Biomed. Opt. Express, 2022, 13(9), 4970-4989
- X. Xu, Z. Deng, D. Sforza, Z. Tong, Y. Tseng, C. Newman, M. Reinhart, P. Tsouchlos, T. Devling, H. Dehghani, I. Iordachita, J. W. Wong, and K. K.-H. Wang. “Characterization of a commercial bioluminescence tomography-guided system for pre-clinical radiation research.” Med. Phys., 2023; 50:6433-6453, Editor’s choice in emerging imaging and therapy modalities
- Z. Deng, X. Xu, H. Dehghani, J. Reyes, L, Zheng, P. T. Tran, and K. K.-H. Wang. "In vivo bioluminescence tomography-guided system for pancreatic cancer radiotherapy research." Biomed. Opt. Express, 2024; 15:4525-4539, DOI: 10.1364/BOE.523916
- B. Deng, Z. Tong, X. Xu, H. Dehghani, and K. K.-H. Wang. "Self-supervised hybrid neural network to achieve quantitative bioluminescence tomography for cancer research." Biomed. Opt. Express, 2024; 15:6211-6227, DOI: 10.1364/BOE.531573.