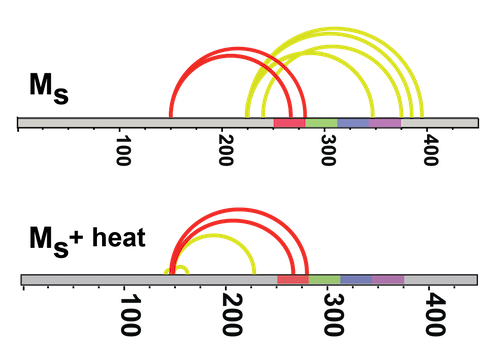
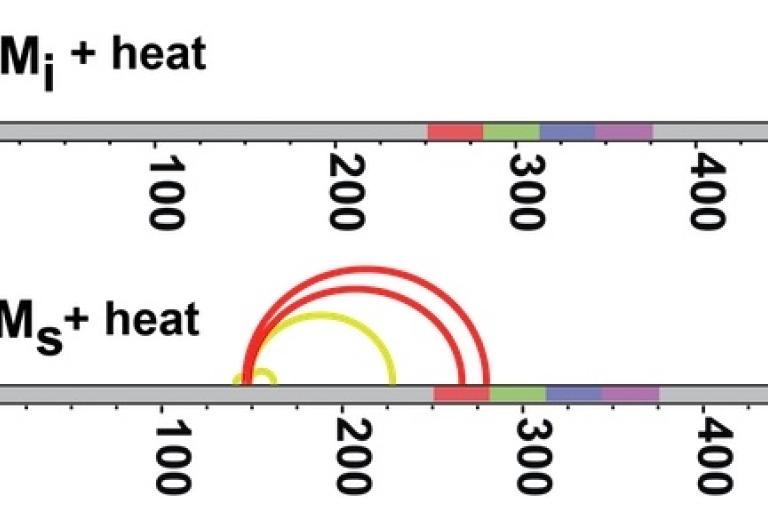
(Above) Crosslinking studies on inert tau monomer (Mi) and tau seeding monomer (Ms) reveal different crosslink patterns.
(Below) Ms crosslinks are also more stable when heated.
We want to understand how the inert form of tau transforms into a pathological shape. A key tool in our research is a technique called cross-linking mass spectrometry (XL-MS), which creates chemical bonds among adjacent portions of the protein to reveal architectural information.
Recently we purified and characterized a conformation of tau monomer that serves as a “seed” that drives aggregation, which we named Ms. Using XL-MS, we found that Ms's shape exposes a portion of the protein that is normally hidden within inert tau monomer (Mi).
We are now studying how different conformations of tau are related to different tauopathies – whether, for instance, tau aggregates in Alzheimer’s disease differ from those in Parkinson’s disease. A clearer understanding of the shapes these conformations adopt will have important implications for the development of better diagnostic and therapeutic strategies.
References
Mirbaha H, et al (2018). Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife:e36584.