Research Overview - Molecular Mechanisms in Cancer
The central goal of the Camacho Lab is to define the molecular mechanisms that drive cancer, with the ultimate aim of identifying novel therapeutic targets. Our research centers on the biology of Poly(ADP-ribose) polymerases (PARPs) and the role of ADP-ribosylation, a reversible post-translational modification that regulates a range of cellular processes, including transcription, gene expression, chromatin structure, and chromatin dynamics.
Our early work in breast cancer uncovered key roles for PARP1 in Estrogen Receptor (ER)-positive disease, including the ADP-ribosylation of the pioneer factor FOXA1 and its downstream effects on estrogen-dependent gene expression. Building on this, we are expanding our efforts to investigate how PARP-mediated ADP-ribosylation regulates gene expression programs and cellular phenotypes in gynecologic cancers, including endometrial and ovarian cancers, disease contexts where PARP function and therapeutic targeting remain incompletely understood.
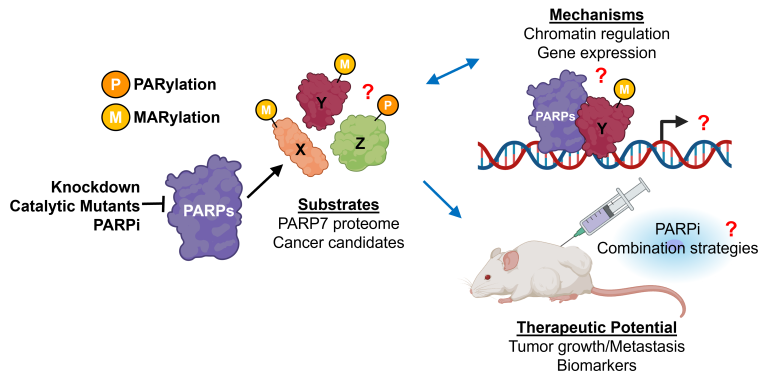
Our current research explores three major areas:
-
Substrates: Identifying PARP substrates whose modification drives cancer-relevant processes such as proliferation, migration, and invasion.
-
Mechanisms: Defining how PARP-mediated ADP-ribosylation regulates chromatin structure, transcriptional networks in gynecologic cancers.
-
Therapeutic Potential: Investigating PARP inhibitor combination strategies, mechanisms of resistance, and biomarkers that predict therapeutic response.
We use a multidisciplinary approach that combines molecular biology, genomics, proteomics, and in vivo models to gain a comprehensive view of PARP function in cancer. Our long-term goal is to translate insights in PARP biology into therapeutic advances for patients with gynecologic malignancies.

Cristel Camacho, Ph.D.
Assistant Professor
The overarching theme of my research interests and goals has focused on understanding key genetic events that lead to cancer in an effort to identify novel targets that will help improve existing therapies. Currently, I am interested in characterizing the molecular basis of breast cancer phenotypes with an emphasis on PARP biology. ADP-ribosylation mediated by PARP-1 is a frequent post-translation modification in eukaryotes shown to play an extensive role in gene regulation by modulating transcription. In Estrogen Receptor (ER)-positive breast cancers, PARP-1 plays a critical role in the direct control of estrogen-dependent gene expression, in part by modulating the activity of the transcription factor FoxA1. The biological consequences of PARP-1-mediated FoxA1 ADP-ribosylation are currently being explored. The long-term goal is to expand our knowledge of the role of PARP-1-mediated ADP-ribosylation in breast cancer biology by employing an array of biochemical, molecular, genomic and proteomic approaches using cell-based and transgenic mouse models of human breast cancer.
Faculty Profile
Marwa Aljardali, M.D.
Postdoctoral Fellow
I joined the Camacho lab in January 2023. I completed my M.D. training in Lebanon in June 2022. As an aspiring obstetrician and gynecologist, I will focus my research on exploring different ways to enhance patient care. My main interests would be investigating novel therapies that could treat gynecologic cancers and finding biomarkers that could be used in clinics to direct the optimal treatment.
Selected Publications
- Aljardali MW, Kremer KM, Parker JE, Fleming E, Chen H, Lea JS, Kraus WL, Camacho CV. Nucleolar Localization of the RNA Helicase DDX21 Predicts Survival Outcomes in Gynecologic Cancers. Cancer Res Commun. 2024 Jun 13;4(6):1495-1504.
- Challa S, Nandu T, Kim HB, Gong X, Renshaw CW, Li WC, Tan X, Aljardali MW, Camacho CV, Chen J, Kraus WL. RACK1 MARylation regulates translation and stress granules in ovarian cancer cells. J Cell Biol. 2025 Feb 3;224(2):e202401101.

Lu Yang, Ph.D.
Postdoctoral Fellow
I joined the Camacho lab in April 2025. My research focuses on elucidating the molecular mechanisms of gene regulation in hormone-driven cancers, particularly emphasizing the role of PARP-mediated ADP-ribosylation. I aim to understand how these pathways influence transcriptional programs and contribute to cancer progression, ultimately informing the development of novel therapeutic strategies.
Selected Publications
- Teng Z, Yang L, Zhang Q, et al. Topoisomerase I is an evolutionarily conserved key regulator for satellite DNA transcription. Nat Commun. 2024;15(1):5151.
- Yang L, Zhang Q, Niu T, Liu H. SET levels contribute to cohesion fatigue. Mol Biol Cell. 2021;32(13):1256-1266.

Shu-Ping Chiu, M.S.
Research Associate
My work contributes to the successful flow of experimentation and research executed by personnel in the lab. I am interested in using my expertise in molecular biology for biochemical assays.
I am an AALAS-certified technician, RALAT, and participate in various studies involving animal models.
I obtained my Master of Science degree from Fu-Jen Catholic University in Taiwan.
Selected Publications
- Chiu SP, Camacho CV, Kraus WL. Development and characterization of recombinant ADP-ribose binding reagents that allow simultaneous detection of mono and poly ADP-ribose. J Biol Chem. 2024 300(9):107609. PMID: 38798442
- Tripathy S, Nagari A, Chiu SP, Nandu T, Camacho CV, Mahendroo M, Kraus WL. Relaxin Modulates the Genomic Actions and Biological Effects of Estrogen in the Myometrium. Endocrinology. 2024 65(11):bqae123. PMID: 39283953
Featured Publications
Challa S, Nandu T, Kim HB, Gong X, Renshaw CW, Li WC, Tan X, Aljardali MW, Camacho CV, Chen J, Kraus WL. RACK1 MARylation regulates translation and stress granules in ovarian cancer cells. J Cell Biol. 2025 Feb 3;224(2):e202401101.
Tripathy S, Nagari A, Chiu SP, Nandu T, Camacho CV, Mahendroo M, Kraus WL. Relaxin Modulates the Genomic Actions and Biological Effects of Estrogen in the Myometrium. Endocrinology. 2024 Sep 26;165(11):bqae123.
Chiu SP, Camacho CV, Kraus WL. Development and characterization of recombinant ADP-ribose binding reagents that allow simultaneous detection of mono and poly ADP-ribose. J Biol Chem. 2024 Sep;300(9):107609.
Aljardali MW, Kremer KM, Parker JE, Fleming E, Chen H, Lea JS, Kraus WL, Camacho CV. Nucleolar Localization of the RNA Helicase DDX21 Predicts Survival Outcomes in Gynecologic Cancers. Cancer Res Commun. 2024 Jun 13;4(6):1495-1504.
Kim DS*, Camacho CV*, Setlem R, Kim K, Malladi S, Hou TY, Nandu T, Gadad SS, Kraus WL. Functional Characterization of lncRNA152 as an Angiogenesis-Inhibiting Tumor Suppressor in Triple-Negative Breast Cancers. Mol Cancer Res. 2022 Nov 3;20(11):1623-1635. *equal contribution
Huang D, Camacho CV, Martire S, Nagari A, Setlem R, Gong X, Edwards AD, Chiu SP, Banaszynski LA, Kraus WL. Oncohistone Mutations Occur at Functional Sites of Regulatory ADP-Ribosylation. Cancer Res. 2022 Jul 5;82(13):2361-2377.
Challa S, Ryu KW, Whitaker AL, Abshier JC, Camacho CV, Kraus WL. Development and characterization of new tools for detecting poly(ADP-ribose) in vitro and in vivo. Elife. 2022 Apr 27;11:e72464.
Gadad SS*, Camacho CV*, Malladi V, Hutti CR, Nagari A, Kraus WL. PARP-1 Regulates Estrogen-Dependent Gene Expression in Estrogen Receptor α-Positive Breast Cancer Cells. Mol Cancer Res. 2021 Oct;19(10):1688-1698. *equal contribution
Challa S, Khulpateea BR, Nandu T, Camacho CV, Ryu KW, Chen H, Peng Y, Lea JS, Kraus WL. Ribosome ADP-ribosylation inhibits translation and maintains proteostasis in cancers. Cell. 2021 Aug 19;184(17):4531-4546.e26.
Full publications list
Lab News
Mary Crowley Cancer Research Foundation Awards $100,000 to Support Endometrial Cancer Research
(January 5, 2026) - Dr. Camacho has received a $100,000 research gift from the Mary Crowley Cancer Research Foundation to support her proposal titled “The Role of PARP7-mediated ADP-ribosylation in Endometrial Cancer Biology.” This funding will accelerate our efforts to understand how PARP7-dependent signaling pathways shape tumor biology and may inform future therapeutic strategies for endometrial cancer.
We are deeply thankful to the Mary Crowley Cancer Research Foundation for their commitment to advancing innovative cancer research.
Award for Best Basic Science Research Presentation
(May 19, 2025) - Camacho Lab postdoctoral fellow Marwa Aljardali, M.D. has been awarded the J.G. Moore Award from the Western Association of Gynecologic Oncology for her abstract "RBN-2397, a PARP7 Inhibitor, Synergizes with Paclitaxel to Inhibit Proliferation and Migration of Ovarian Cancer Cells," which was presented at the WAGO 2024 Annual Meeting. The J.G. Moore Award recognizes the best basic science research presentation by a resident or fellow.
Research publicationJoin the Lab!
Postdoctoral training opportunity
A postdoctoral training position is available to study gene regulation in breast cancer. The Camacho Lab has several exciting projects related to hormone signaling and gene regulation, focusing on transcription and nuclear endpoints of cellular signaling pathways. We are interested in a wide variety of model systems and experimental approaches, including biochemistry, molecular biology, animal models, genomics, proteomics, bioinformatics, and computational biology. Projects in the lab are focused on signal-regulated transcription in the chromatin environment of the nucleus, targeting the estrogen and nuclear NAD+ signaling pathways, PARPs, and transcription factors in breast cancer biology.
Candidates must hold a recent Ph.D. and/or M.D. degree. Experience in Biochemistry, Molecular Biology, Genomics, and/or Computational Biology, leading to publication in peer-reviewed journals is recommended.
Contact Us
Email Address: cristel.camacho@utsouthwestern.edu
Phone Number: 214-648-2442
Physical Address:
5323 Harry Hines Blvd.
Building J, Room J7.116
Dallas, TX 75390-85