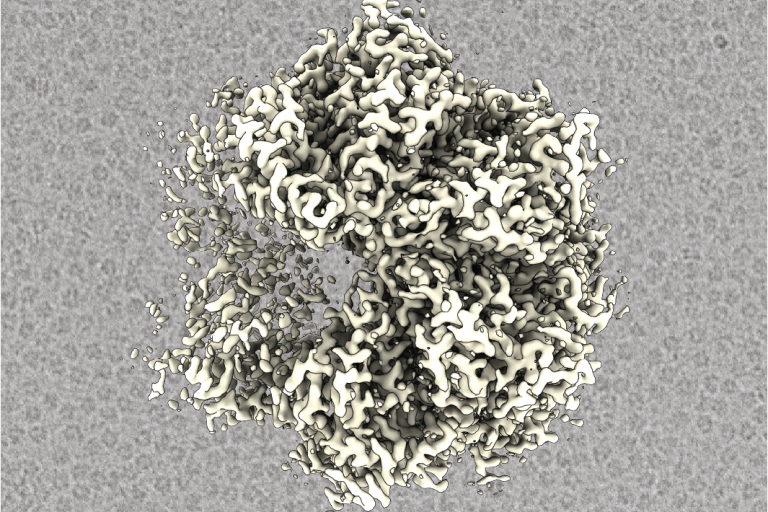
Building on our mechanistic studies of ribosome assembly, we are investigating how small molecules can selectively inhibit key enzymatic steps in this process to block cancer cell growth. Our current focus is NVL, a conserved AAA⁺ ATPase that functions as a molecular machine driving 60S subunit maturation. In close collaboration with the Nijhawan and DeBrabander labs, we use cryo-electron microscopy, biochemical assays, and chemical biology approaches to define the molecular mechanism of NVL and its inhibition by a class of potent antiproliferative compounds. In its active, hexameric form, NVL acts as a protein unfoldase, translocating peptide through a central channel to structurally remodel and release ribosome assembly factors from pre-ribosomal particles. We are working to identify its physiological substrate and the molecular events that govern substrate recognition and engagement, critical steps for understanding how these inhibitors disrupt NVL function to stop malignant cell growth. Together, these structural and biochemical insights form a tightly integrated effort with our collaborators’ medicinal chemistry programs, illustrating how fundamental discoveries in ribosome biology can directly power the development of new therapeutic strategies against cancer.