Meet the PI

Hirofumi Fujita, M.D., Ph.D.
Principal Investigator | Assistant Professor
Department of Neurology
Hirofumi received his M.D. and Ph.D. from Tokyo Medical and Dental University, Tokyo, Japan. His graduate works in Izumi Sugihara laboratory focused on molecular segmentation of the cerebellar cortex in which he identified how the spatial rearrangement of the cerebellar neurons during early development forms distinct zones of the cerebellum. For postdoctoral training, he moved to the United States and joined the Sascha du Lac laboratory at Salk Institute, CA, and Johns Hopkins University, MD. He identified that each cerebellar cortical zone is connected to their private target neurons in the cerebellar nuclei, and the distinction in these cerebellar nuclear neurons underlies diverse patterns of cerebellar outputs to the rest of the brain. His work has been providing novel insights into the circuit frameworks of how the cerebellum can be involved in so many distinct motor and nonmotor functions. In 2022, Hirofumi joined the Department of Neurology.
Research Interests
The cerebellum helps the healthy functioning of the brain and compensates for the diseased brain functions through its massive interconnections with many forebrain and brainstem regions. The Fujita lab utilizes disease model mice, including spinocerebellar ataxia and autism, and human samples to study 1) input-output organization of how the cerebellum is connected with the regions/neurons in the forebrain and brainstem, 2) mechanisms of how the cerebellum influences the formation and maintenance of healthy forebrain and brainstem circuits.
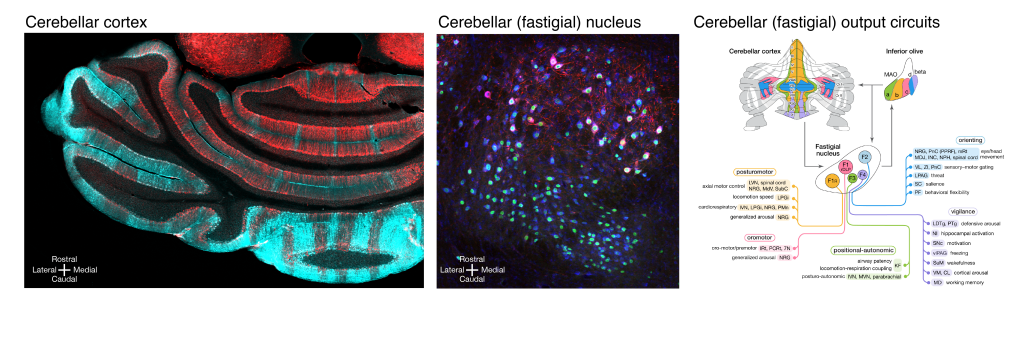 Left, molecular segmentation of the cerebellum into multiple striped zones – Aldoc in cyan, Nefh in red. Middle, cerebellar nucleus, the fastigial nucleus in this example, is comprised of heterogeneous types of neurons that differ in size, distribution, and molecular expression – Nefh in red, Vglut2Cre;Sun1/sfGFP in green, Nissl in blue. Right, diagram of modular cerebellar output circuits formed by distinct neurons in the cerebellar vermal cortex, the cerebellar fastigial nucleus, and the output regions.
Left, molecular segmentation of the cerebellum into multiple striped zones – Aldoc in cyan, Nefh in red. Middle, cerebellar nucleus, the fastigial nucleus in this example, is comprised of heterogeneous types of neurons that differ in size, distribution, and molecular expression – Nefh in red, Vglut2Cre;Sun1/sfGFP in green, Nissl in blue. Right, diagram of modular cerebellar output circuits formed by distinct neurons in the cerebellar vermal cortex, the cerebellar fastigial nucleus, and the output regions. Featured Publications
Catecholamine signaling that modulates cerebellar operations in cognition.
Fujita H, Carlson ES. Neuropsychopharmacology. 2021;46(1):248-249.
Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits.
Kelly E, Meng F, Fujita H, Morgado F, Kazemi Y, Rice LC, Ren C, Escamilla CO, Gibson JM, Sajadi S, Pendry RJ, Tan T, Ellegood J, Basson MA, Blakely RD, Dindot SV, Golzio C, Hahn MK, Katsanis N, Robins DM, Silverman JL, Singh KK, Wevrick R, Taylor MJ, Hammill C, Anagnostou E, Pfeiffer BE, Stoodley CJ, Lerch JP, du Lac S, Tsai PT. Nat Neurosci. 2020;23(9):1102-1110.
Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis.
Fujita H, Kodama T, du Lac S. Elife. 2020;9:e58613.
Lobular homology in cerebellar hemispheres of humans, non-human primates and rodents: a structural, axonal tracing and molecular expression analysis.
Luo Y, Fujita H, Nedelescu H, Biswas MS, Sato C, Ying S, Takahashi M, Akita K, Higashi T, Aoki I, Sugihara I. Brain Struct Funct. 2017;222(6):2449-2472.
Detailed expression pattern of aldolase C (Aldoc) in the cerebellum, retina and other areas of the CNS studied in Aldoc-Venus knock-in mice.
Fujita H, Aoki H, Ajioka I, Yamazaki M, Abe M, Oh-Nishi A, Sakimura K, Sugihara I. PLoS One. 2014;9(1):e86679.
Clustered fine compartmentalization of the mouse embryonic cerebellar cortex and its rearrangement into the postnatal striped configuration.
Fujita H, Morita N, Furuichi T, Sugihara I. J Neurosci. 2012;32(45):15688-15703.
Contact Us
- Hirofumi Fujita
- UT Southwestern Medical Center
- 6000 Harry Hines Blvd, NL building 9th floor, Room 118B, Dallas, TX 75235