Our research efforts are focused on developing novel computational algorithms, computational models, and computational pipelines for big, heterogeneous biomedical and clinical data. Here, we briefly present some examples.
The DeepEar Project
The detection of congenital auricular deformity within 6 months after birth is necessary to avoid later surgical interventions. Currently, there are no reliable objective methods to detect ear deformity; subjective assessment is the only way. Therefore, we developed a deep learning model using a transfer learning technique to classify ears from 2D photographs as normal or deformed with high accuracy. The methodology is depicted in the graphic below from our manuscript Identifying ear abnormality from 2D photographs using convolutional neural networks published in Scientific Reports in 2019 (authors Rami R. Hallac, Jeon Lee, Mark Pressler, James R. Seaward, and Alex A. Kane).
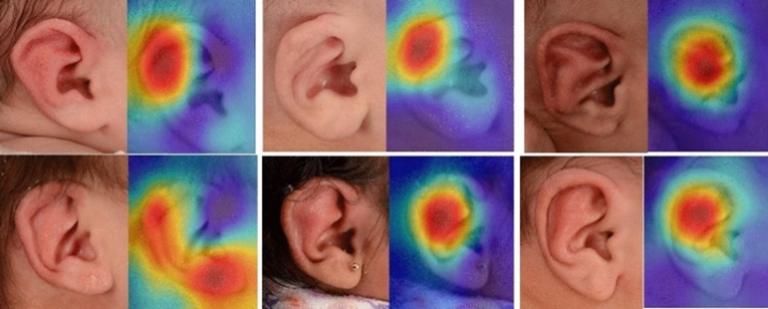 Infant ears with corresponding deep ear heat maps
Infant ears with corresponding deep ear heat maps 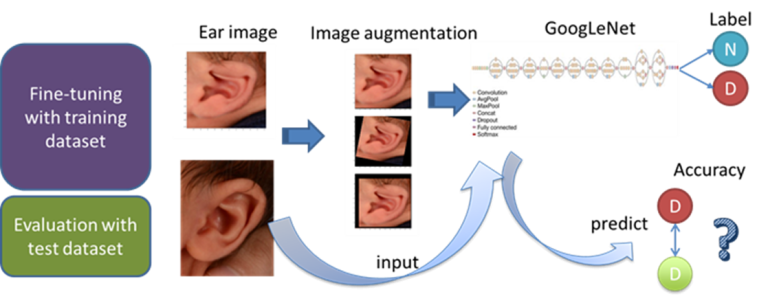 Technical diagram of deep ear project
Technical diagram of deep ear project ECMO Injury Prediction Project
Brain injury is a significant source of morbidity and mortality for pediatric patients treated with Extracorporeal Membrane Oxygenation (ECMO). To predict brain injury in pediatric ECMO-supported patients, we developed a hybrid deep learning model consisting of a recurrent neural network (LSTM) and a convolutional neural network (CNN). Taking time series data comprised of physiological features, markers of end-organ perfusion, acid-base homeostasis, vasoactive infusions, markers of coagulation, and ECMO-machine factors, our deep learning model demonstrated accurate predictions that surpassed clinician judgments regarding pediatric patients on ECMO that were more likely to develop brain injury. Details of this study can be found within our manuscript Neural networks to predict radiographic brain injury in pediatric patients treated with extracorporeal membrane oxygenation published in 2020 in the Journal of Clinical Medicine (authors Neel Shah, Abdelaziz Farhat, Jefferson Tweed, Ziheng Wang, Jeon Lee, Rafe McBeth, Michael Skinner, Fenghua Tian, Ravi Thiagarajan, and Lakshmi Raman).
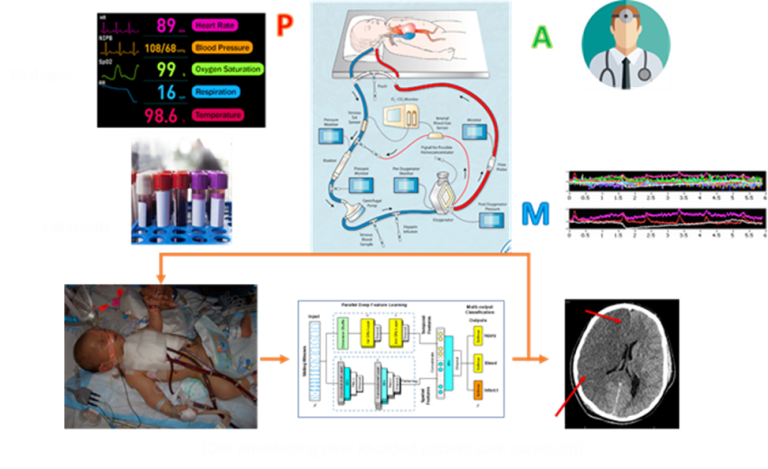 ECMO Injury Prediction Project diagram
ECMO Injury Prediction Project diagram HIV-1 Proviral Expression Project
The impact of the HIV-1 integration site on proviral transcription and expression is not well understood and requires the analysis of multiple genomic datasets for thousands of proviral integration sites across the human genome. We generated and combined large-scale datasets, including epigenetics, transcriptome, and 3D genome architecture to interrogate the chromatin states, transcription activity, and nuclear sub-compartments around HIV-1 integrations in Jurkat CD4+ T-cells to decipher human genome regulatory features shaping proviral transcription. Using machine learning approaches, we defined the nuclear sub-compartments and chromatin states that shape genomic architecture, transcriptional activity, and nucleosome density of neighboring regions around the integration site. These were all additive features influencing HIV-1 expression. Based on this integrative genomics approach, we can now introduce HIV-1 at targeted genomic locations having known regulatory features and study how such integrations influence viral expression. More details in our manuscript Decoding Human Genome Regulatory Features That Influence HIV-1 Proviral Expression and Fate Through an Integrated Genomics Approach published in Bioinformatics and Biology Insights in 2022 (authors Holly Ruess, Jeon Lee, Carlos Guzman, Venkat S. Malladi, and Iván D'Orso).
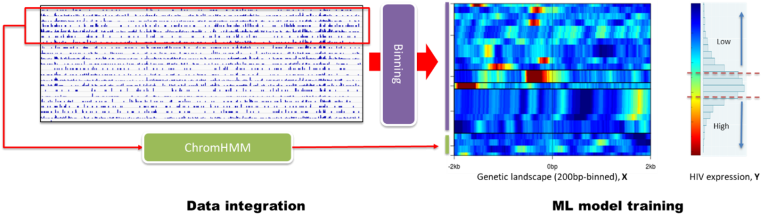 Using data integration and machine learning to define expression and fate of HIV-1 proviral transcripts
Using data integration and machine learning to define expression and fate of HIV-1 proviral transcripts Human Prostate Anatomy Project
The cellular origins of benign prostatic hyperplasia (BPH) and prostate cancer are still unknown. To properly define human prostate cellular anatomy and create a baseline for understanding the cellular origins of disease, we performed single-cell RNA sequencing (scRNA-seq) on single cells from five young adult human prostates. After multiple scRNA-seq data normalization and integration, two unrecognized epithelial cell types were newly identified, and previously unknown markers were derived for established cell types. Details can be found in our manuscript A cellular anatomy of the normal adult human prostate and prostatic urethra published in Cell Reports in 2018 (authors Gervaise H. Henry, Alicia Malewska, Diya B. Joseph, Venkat S. Malladi, Jeon Lee, Jose Torrealba, Ryan J. Mauck, Jeffrey C. Gahan, Ganesh V Raj, Claus G. Roehrborn, Gary C. Hon, Malcolm P. MacConmara, Jeffrey C. Reese, Ryan C. Hutchinson, Chad M. Vezina, and Douglas W. Strand).
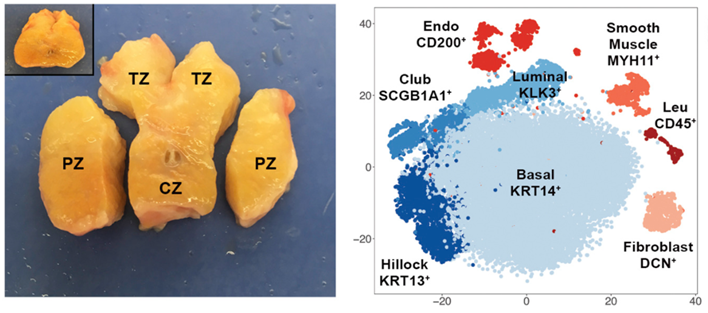 Defining human prostate cancer anatomy by single-cell RNA sequencing
Defining human prostate cancer anatomy by single-cell RNA sequencing Prediction of Mechanism of Action Project
Assigning the accurate mechanism of action to botanicals, natural products, and synthetic chemicals continues to be a major challenge. We integrated gene expression-based (FUSION) and high content imaging-based (Cytological Profiling, CP) screening platforms using Similarity Network Fusion (SNF) that resulted in a novel, improved framework for functional annotation of natural products. We validated the utility of this computational prediction by confirming and demonstrating that natural product factions containing Trichostatin A were clustered with pure Trichostatin A and other HDAC inhibitors. This work is described in our manuscript High-throughput functional annotation of natural products by integrated activity profiling published in PNAS in 2022 (authors Suzie K. Hight, Kenji L. Kurita, Elizabeth A. McMillan, Walter Bray, Trevor N. Clark, Anam F. Shaikh, F. P. Jake Haeckl, Fausto Carnevale-Neto, Scott La, Akshar Lohith, Rachel M. Vaden, Jeon Lee, Shuguang Wei, R. Scott Lokey, Michael A. White, Roger G. Linington, and John B. MacMillan).
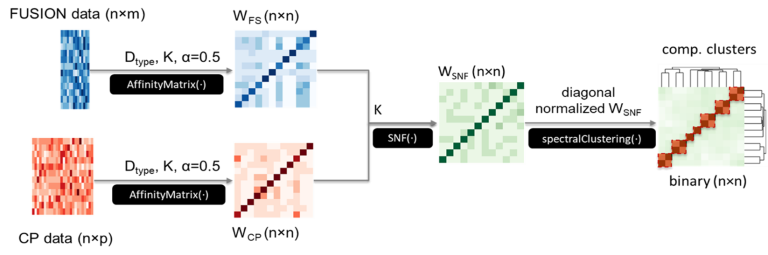 Prediction of Mechanism of Action Project diagram
Prediction of Mechanism of Action Project diagram Associations between Brain Morphometry and Neural Oscillations
It is critical to understand how neural circuits underlie complex behaviors in health and disease. A prerequisite to this goal is to gain greater insights into the variables affecting the signals used for investigating these neural circuits. While most investigators recognize significant variability in these signals, the sources of this variability are not well understood. In this project, collaborating with Pouratian lab, we have been investigating associations between brain morphometry and brain signals, especially neuronal oscillations, which are recorded via local field potential (LFP) from deep within the brain using depth electrodes or deep brain stimulation electrodes, or via electrocorticography (ECoG) from the surface of the brain. We hypothesize that loss of neurons either locally (as may be reflected in measures of cortical thickness on sMRI) or from distant brain regions (as may be reflected in tractography measures on DTI) can affect neural oscillations patterns.
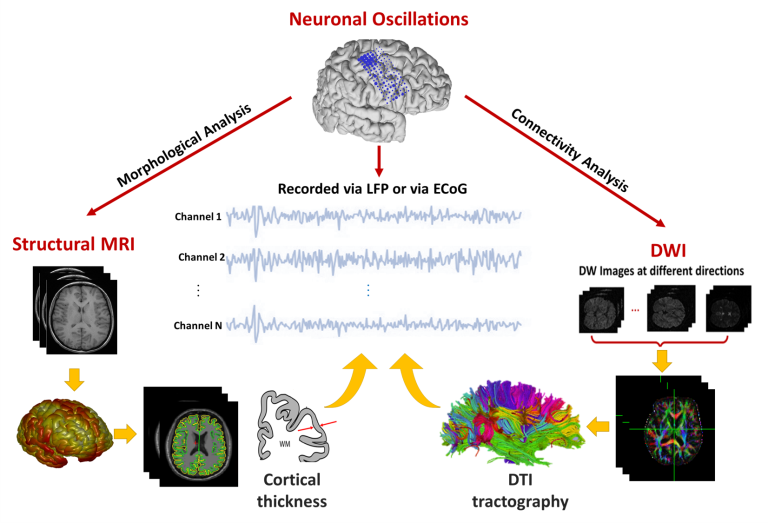 Brain morphometry and neural oscillations project diagram
Brain morphometry and neural oscillations project diagram