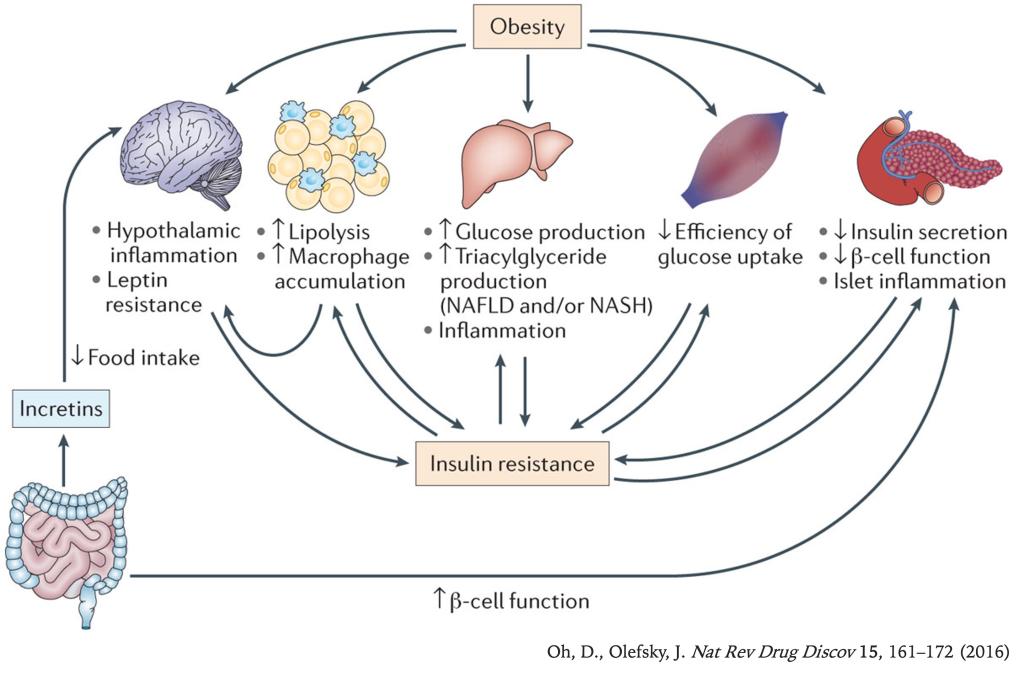
The prevalence of obesity and type 2 diabetes (T2D) is increasing worldwide, and these two metabolic disorders are closely linked. Lifestyle modification, including weight loss and exercise, are effective treatments for T2D, but, unfortunately, most patients are unsuccessful at maintaining durable weight reduction and recidivism is all too common. Therefore, safe and efficacious drugs are required for successful treatment of T2D in a large proportion of subjects.
Seven transmembrane receptors (7TMRs), often termed G protein-coupled receptors (GPCRs), are the most common targets of therapeutic drugs today. Targeting GPCRs in metabolic tissues - such as adipose, liver, muscle, pancreatic islet, immune, and central nervous tissues - has emerged as a key strategy for the development of anti-diabetic compounds.
In the Oh Lab, we are interested in studying the role of GPCRs in obesity, inflammation, and T2D.
Currently, we are primarily working on GPR120, GPR92, and GPR84.
1. Revisiting PPAR gamma as a new friend of GPR120 in the treatment of metabolic disorders.
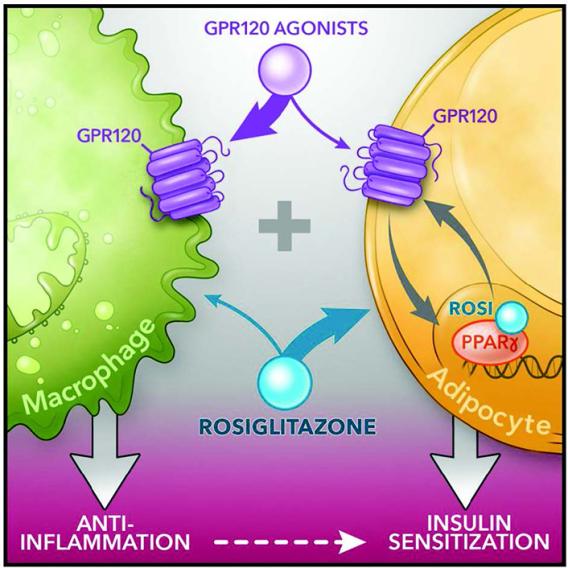 Cell Metab. 2020 Jun 2; 31(6): 1173–1188.e5.
Cell Metab. 2020 Jun 2; 31(6): 1173–1188.e5. G Protein-coupled receptor 120 (GPR120; fatty acid receptor 4, FFAR4) and PPARγ agonists both lead to anti-inflammatory and insulin sensitizing effects despite signaling through distinct pathways. We recently reported the overarching idea that these 2 pathways are interactive. Specifically, treatment of obese mice with the PPARγ agonist rosiglitazone (a thiazolidinedione, TZD) in combination with the GPR120 agonist compound A synergistically improves glucose tolerance and insulin sensitivity. We have deconvoluted the mechanisms underlying this feed-forward effect in the study. Taken together, our study shows that low dose TZD administration, in combination with GPR120 agonists, produces additive beneficial effects on glucose tolerance and insulin sensitivity without the undesirable adverse effects of TZD. Our study suggests potential value of combination PPARγ and GPR120 agonists to treat metabolic disease.
2. GPR92 activation in islet macrophages to control beta cell function in a diet-induced obesity
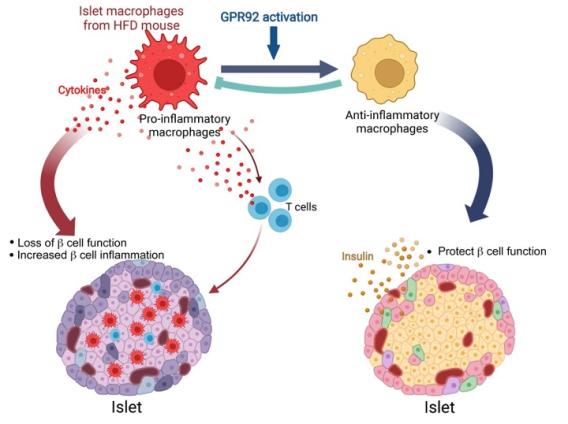 J Clin Invest . 2022 Nov 1;132(21):e160097.
J Clin Invest . 2022 Nov 1;132(21):e160097. Our study revealed that GPR92, expressed in islet macrophages, is modulated by dietary interventions in metabolic tissues. Therefore, we aimed to define the role of GPR92 in islet inflammation by using a high-fat diet-induced (HFD-induced) obese mouse model. GPR92-KO mice exhibited glucose intolerance and reduced insulin levels - despite the enlarged pancreatic islets - as well as increased islet macrophage content and inflammation level compared with WT mice. These results indicate that the lack of GPR92 in islet macrophages can cause β cell dysfunction, leading to disrupted glucose homeostasis. Alternatively, stimulation with the GPR92 agonist farnesyl pyrophosphate results in the inhibition of HFD-induced islet inflammation and increased insulin secretion in WT mice, but not in GPR92-KO mice. Thus, our study suggests that GPR92 can be a potential target to alleviate β cell dysfunction via the inhibition of islet inflammation associated with the progression of diabetes.
3. GPR84-mediated signal transduction affects brown adipocyte activity
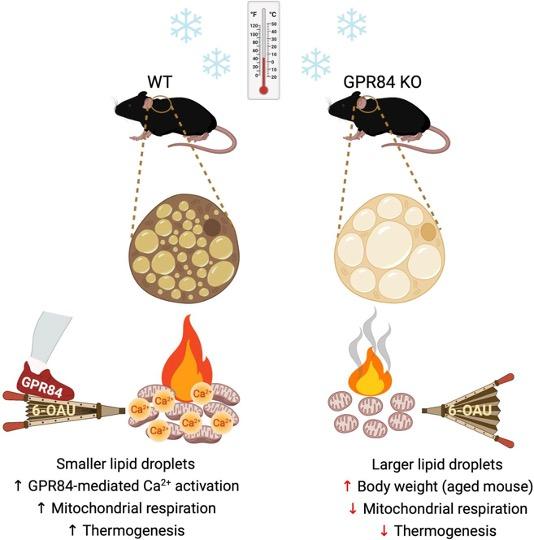 J Clin Invest . 2023 Dec 15;133(24):e168992.
J Clin Invest . 2023 Dec 15;133(24):e168992. The G protein-coupled receptor 84 (GPR84), a medium-chain fatty acid receptor, has garnered attention because of its potential involvement in a range of metabolic conditions. However, the precise mechanisms underlying this effect remain elusive. Our study has shed light on the pivotal role of GPR84, revealing its robust expression and functional significance within brown adipose tissue (BAT). Mice lacking GPR84 exhibited increased lipid accumulation in BAT, rendering them more susceptible to cold exposure and displaying reduced BAT activity compared with their WT counterparts. Our in vitro experiments with primary brown adipocytes from GPR84-KO mice revealed diminished expression of thermogenic genes and reduced O2 consumption. Furthermore, the application of the GPR84 agonist 6-n-octylaminouracil (6-OAU) counteracted these effects, effectively reinstating the brown adipocyte activity. These compelling in vivo and in vitro findings converge to highlight mitochondrial dysfunction as the primary cause of BAT anomalies in GPR84-KO mice. The activation of GPR84 induced an increase in intracellular Ca2+ levels, which intricately influenced mitochondrial respiration. By modulating mitochondrial Ca2+ levels and respiration, GPR84 acts as a potent molecule involved in BAT activity. These findings suggest that GPR84 is a potential therapeutic target for invigorating BAT and ameliorating metabolic disorders.