Our laboratory investigates the molecular mechanisms underlying the pathogenesis of sepsis. We use the heart as a model, since cardiac dysfunction is a vital component of multi-organ failure during sepsis.
By combining approaches of molecular biology, biochemistry, histology, and physiology assessments, we aim to define the role of mitochondrial abnormality and production of reactive oxygen species (mtROS) in inciting cardiac inflammation and autophagy in preclinical models.
To explore therapeutic options, we are evaluating whether targeted inhibition of mtROS provides a powerful therapeutic potential for sepsis.
A pathological consequence of damage in mitochondria and in mitochondrial DNA (mtDNA) is the release of free mtDNA fragments into cytoplasm, and later to extracellular spaces. These mtDNA fragments function as danger-associated molecular patterns to induce inflammation via toll-like receptor 9 (TLR9). Further, a complex formed by the receptor for advanced glycation end-products and mitochondrial transcription factor A is a critical component to facilitate the signal transduction.
Our current investigation indicates that this signaling pathway is dependent on mitochondrial reactive oxygen species, and is used in cardiac inflammation in response to sepsis.
Future studies will determine how cardiac inflammation is regulated via different TLR pathways, spatially and temporally, or by their crosstalk. These studies will help clarify the pathological mechanisms underlying sepsis-induced cardiac dysfunction.
Autophagy, a lysosome-dependent process of removing damaged proteins and organelles, is stimulated during sepsis.
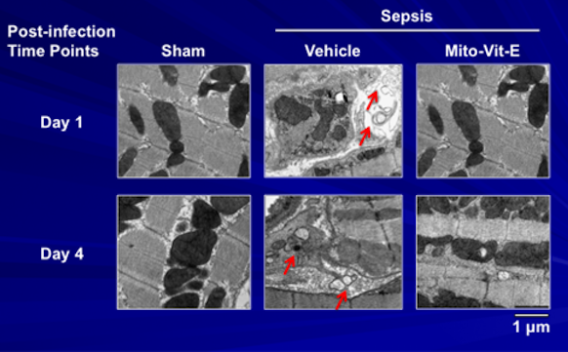
We recently detected a significant accumulation of autophagosomes in the heart tissue of septic animals; this change was alleviated by mitochondria-targeted vitamin E (Mito-Vit-E).
Our ongoing work aims to identify the molecular pathways of mtROS-dependent autophagy and to determine the pathological role of autophagy in sepsis outcomes using both in vitro and in vivo models.
Among many intracellular players that contribute to the pathogenesis of sepsis, oxidative stress is well recognized as a major promoter of the condition. However, clinical trials of antioxidant therapy have yielded inconsistent efficacy at maximum tolerated doses.
One limitation of the currently available antioxidants is that they are globally acting agents, and lower antioxidant efficacy is speculated as possible reason for the failures. For the same reason, these antioxidants are often administered prophylactically as preventive agents rather than therapeutic regimens.
To date, the massive oxidative injury induced by sepsis remains untreatable and is a major obstacle in the field.
Because mitochondria are the main organelles generating reactive oxygen species, concentrating antioxidant activities specifically in mitochondria may enhance both efficacy and safety. We, and others, hypothesize that the newly developed mitochondria-targeted antioxidants (MTAs) may offer an opportunity for the development of a promising therapy for sepsis.
In a pneumonia-related sepsis model, our laboratory recently showed that mitochondria-targeted vitamin E protected cardiac mitochondrial structure and function, attenuated myocardial inflammation, and improved heart performance.
We are currently evaluating the therapeutic value of MTAs as a post-injury intervention using sepsis animal models. These studies will lay a solid preclinical groundwork for translation of new drugs into clinical applications.
Join Our Lab
Be part of the great impact we're having on science and medical care across the globe.We are looking for dynamic and motivated researchers, explore available opportunities at the link below.