In the Suleiman lab, we focus on studying the podocyte biology, specifically the actin dynamics and cytoskeleton. Our research includes examining the balance of Rac and RhoA, two members of the Rho small GTPases, in both healthy and diseased kidneys. We utilize a variety of super-resolution microscopy techniques, such as STORM, Airyscan, and N-SPARK, to image the podocyte cytoskeleton and investigate podocyte contractility. We also study the formation of sarcomere-like structures (SLSs) in glomerular pathological conditions like focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD). These SLSs are similar to the sarcomeres found in muscle cells and are believed to be a crucial part of the podocyte's injury and repair process. We have developed a method to visualize the actin cytoskeleton in entire glomeruli using focused ion beam scanning electron microscopy (FIB-SEM), enabling us to investigate the actin dynamics of podocytes in vivo, under both healthy and diseased conditions.
We developed a new ex vivo system that allows us to study primary podocytes freshly isolated from isolated glomeruli and seeded on defined substrate microprinted on hydrogel system. Excitingly, this system has allowed us to observe phenomena in primary podocytes that we had not previously seen. It also provides a framework for studying aspects of podocyte mechanobiology. We electroporated isolated glomeruli and primary podocytes with various types of plasmids, opening up the opportunity to directly study podocyte biology in its native environment.
We utilize super-resolution microscopy to analyze alterations in the molecular structure of the glomerular basement membrane (GBM) in both healthy and diseased states, including diabetic nephropathy (DN) and transplant glomerulopathy (TG). Additionally, we have developed new methods that enable us to conduct electron microscopy on the section following super-resolution imaging.
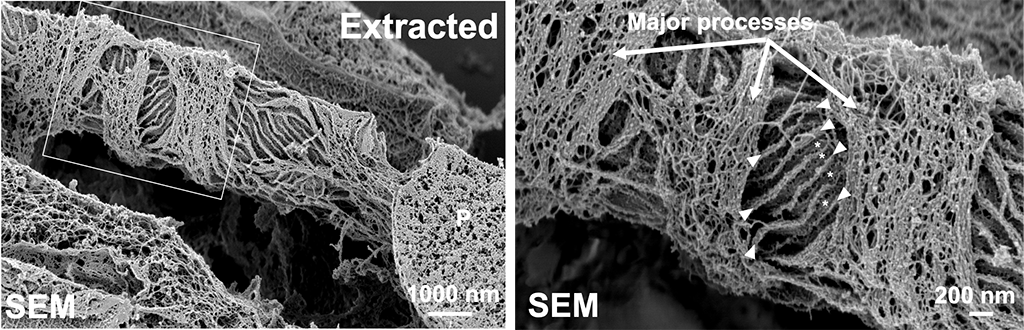
Development of a novel method to image the podocyte cytoskeletal network in 3D.
Using a novel imaging approach that combines with advanced electron microscopic techniques and deep learning, I, and for the first time, was able to visualize the cytoskeleton of podocytes in 3D in their native environment, the glomerulus. I was able to directly image the connections between the actin cables in the foot processes and the adjacent slit diaphragms and identified newly described thick actin bundles around the cell body and the major processes. Moreover, I identified thick actin condensations in the effaced areas in three mouse models for podocyte injury. This work will change our views about the biomechanics of podocytes in both health and disease conditions. Using this technique, we will be able to dissect the role of actin cytoskeleton proteins in actin assembly in the effaced areas after podocyte injury.
- Qu, C, Roth R, Loitman C, Hammad D, Genin GM, Miner JH, Suleiman HY. 3D Visualization of the Podocyte Actin Network using Integrated Membrane Extraction, Electron Microscopy, and Deep Learning. bioRxiv, 2021: p. 2021.01.29.428712. [Preprint].
- Qu, C, Roth R, Puapatanakul P, Loitman C, Hammad D, Genin GM, Miner JH, Suleiman HY. 3D Visualization of the Podocyte Actin Network using Integrated Membrane Extraction, Electron Microscopy, and Machine Learning. J Am Soc Nephrol. 2022 Jan;33(1):155-173. PMC8763187.
Development of a novel system to study primary podocyte cytoskeletal network ex vivo and model aspects of podocyte mechanobiology.
We designed and generated the first ex vivo micropatterned hydrogel system that allows us to study the primary podocytes directly after they spread out of isolated glomeruli. This system utilizes extracellular matrix substrate microprinted on a hydrogel with specific stiffness, which allows us to study the primary podocyte actin cytoskeleton in various conditions by altering both the substrate type and concentration as well as by altering the hydrogel stiffness. Surprisingly, this system allowed us, and for the first time, to observe the primary podocytes upregulating a mat of the mechanosensitive sarcomere-like structures (SLSs).
- Jiang S, Alisafaei F, Yuan H, Hu Y, Peng X, Qu C, Puapatanakul P, Jain S, Miner JH, Genin GM, Suleiman HY. An ex vivo culture model of kidney podocyte injury reveals mechanosensitive, synaptopodin-templating, sarcomere-like structures. Sci. Adv., 2022 Sep;35(8): eabn6027. PMC9432837.