Our Published Work
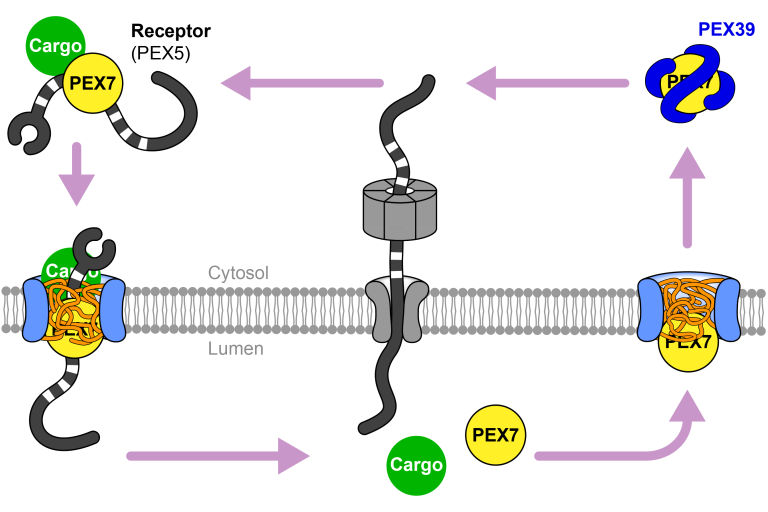
Import mechanism of peroxisomal proteins with an N-terminal signal sequence
Skowyra ML and Rapoport TA, Nat Cell Biol 2025
Peroxisomal proteins with an N-terminal import signal are recognized in the cytosol by an adapter called PEX7, which then hijacks an import receptor to shuttle the cargo through a nuclear pore-like conduit into the organelle. After releasing cargo in the peroxisomal lumen, PEX7 moves back into the conduit and is extracted into the cytosol by a chaperone called PEX39. The chaperone then reloads PEX7 with new cargo and receptor molecules to start another round of translocation.
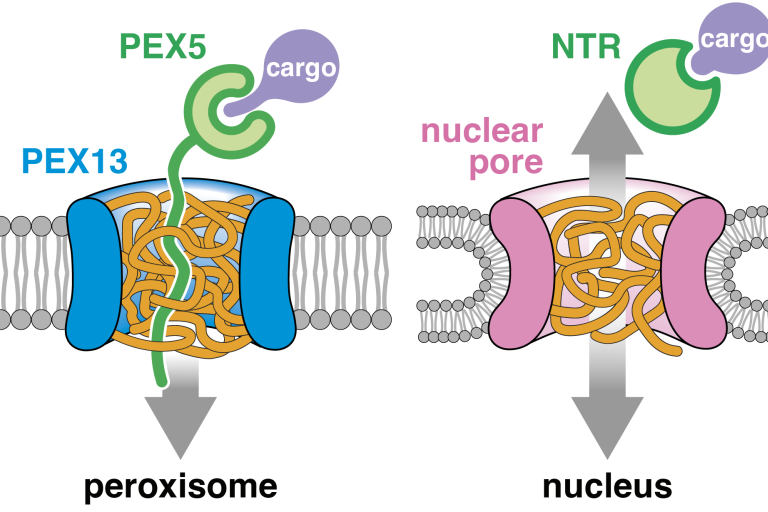
Protein import into peroxisomes occurs through a nuclear pore-like phase
Gao Y*, Skowyra ML*, Feng P, Rapoport TA, Science 2022
Peroxisomal proteins are imported from the cytosol in a folded state by an unknown mechanism. We show that a peroxisomal protein called PEX13 phase-separates in the membrane into a dense meshwork. Mobile import receptors can diffuse through this barrier to bring cargo into the organelle, while other proteins cannot. This mechanism resembles transport through the nuclear pore.
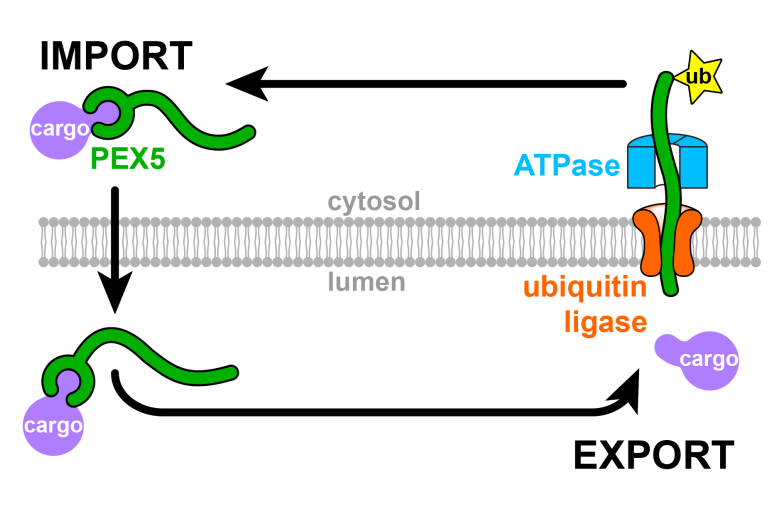
PEX5 translocation into and out of peroxisomes drives matrix protein import
Skowyra ML and Rapoport TA, Mol Cell 2022
Peroxisomal proteins are imported from the cytosol by a receptor called PEX5. We developed a cell-free system based on frog egg extract to show that PEX5 shuttles proteins completely into the peroxisomal lumen. PEX5 is then exported back into the cytosol by a membrane-embedded ubiquitin ligase.
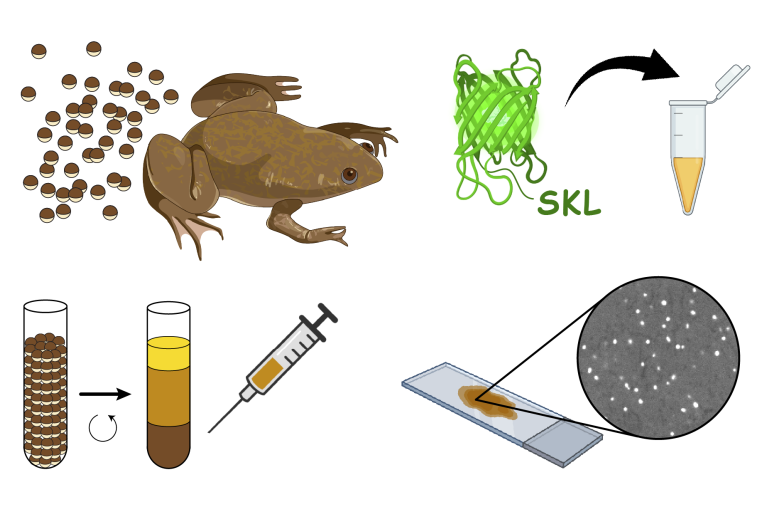
Cell-free reconstitution of peroxisomal matrix protein import using Xenopus egg extract
Skowyra ML and Rapoport TA, STAR Protoc 2023
To study peroxisomal protein import in vitro, we have developed a cell-free system based on a cytoplasmic extract from the eggs of the African clawed frog Xenopus laevis.
Triggered recruitment of ESCRT machinery promotes endolysosomal repair
Skowyra ML, Schlesinger PH, Naismith TV, Hanson PI, Science 2018
Structure and membrane remodeling activity of ESCRT-III helical polymers
McCullough J, Clippinger AK, Talledge N, Skowyra ML, Saunders MG, Naismith TV, Colf LA, Afonine P, Arthur C, Sundquist WI, Hanson PI, Frost A, Science 2015
Mycobacterium tuberculosis Type VII secretion system effectors differentially impact the ESCRT endomembrane damage response
Mittal E, Skowyra ML, Uwase G, Tinaztepe E, Mehra A, Köster S, Hanson PI, Philips JA, mBio 2018
Model-driven mapping of transcriptional networks reveals the circuitry and dynamics of virulence regulation
Maier EJ, Haynes BC, Gish SR, Wang ZA, Skowyra ML, Marulli AL, Doering TL, Brent MR, Genome Res 2015
Cryptococcus neoformans dual GDP-mannose transporters and their role in biology and virulence
Wang ZA, Griffith CL, Skowyra ML, Salinas N, Williams M, Maier EJ, Gish SR, Liu H, Brent MR, Doering TL, Eukaryot Cell 2014
Identification of the galactosyltransferase of Cryptococcus neoformans involved in the biosynthesis of basidiomycete-type glycosylinositolphosphoceramide
Wohlschlager T, Buser R, Skowyra ML, Haynes BC, Henrissat B, Doering TL, Künzler M, Aebi M, Glycobiology 2013
Unusual galactofuranose modification of a capsule polysaccharide in the pathogenic yeast Cryptococcus neoformans
Heiss C, Skowyra ML, Liu H, Klutts JS, Wang Z, Williams M, Srikanta D, Beverley SM, Azadi P, Doering TL, J Biol Chem 2013
Toward an integrated model of capsule regulation in Cryptococcus neoformans
Haynes BC, Skowyra ML, Spencer SJ, Gish SR, Williams M, Held EP, Brent MR, Doering TL, PLoS Pathog 2011
A xylosylphosphotransferase of Cryptococcus neoformans acts in protein O-glycan synthesis
Reilly MC, Aoki K, Wang ZA, Skowyra ML, Williams M, Tiemeyer M, Doering TL, J Biol Chem 2011