The Greenberg Lab is focused on the development of novel therapeutic approaches to combat infectious diseases.
Our specific projects are listed below.
Alternating Magnetic Field (AMF)
Prosthetic joint infection is a major challenge in the field of joint arthroplasty due to the presence of bacterial biofilm on infected implants. While the risk of developing a post-surgical prosthetic joint infection is estimated to occur in 1-2 percent of patients, the current treatment paradigm of antibiotics and prosthetic joint resection/replacement is prolonged, invasive, and expensive. The most prevalent Gram-negative and Gram-positive organisms isolated from these infected implants are Pseudomonas aeruginosa and Staphylococcus aureus. In 2017, the WHO released a list of the most dangerous superbugs, with P. aeruginosa classified as a critical priority and S. aureus as a high priority for new antimicrobial development. We utilize a multidisciplinary approach, combining the expertise of our lab and close collaboration with Rajiv Chopra at Solenic Medical to utilize antibiotics and an Alternating Magnetic Fields (AMF) to disrupt implant-associated biofilm.
Speaker 1 (00:00:00.05)
Prosthetic joint replacement has been transformational in medicine, although there are complications, including infection. And when a joint gets infected frequently we have the formation of biofilm, this thick aggregation of bacteria which reach a certain density and produce these extracellular substances that create a thick matrix on the surface. This has a couple problems associated with it. One, inflammatory cells can't reach their target the pathogen. And the antibiotics we use to treat infections have a hard time reaching their target as well.
Speaker 1 (00:00:37.23)
So what can we do to treat these infections? We've been thinking about a non invasive way where we would wrap a solenoid coil around the implant and when you put an electric current through it, it creates alternating magnetic fields and anything that's conductive inside this environment will actually create heat. And the question we wanted to answer was whether heating just the surface of a prosthetic implant was enough to actually melt away the biofilm, rendering the pathogen susceptible both to inflammatory cells and antibiotics and giving us the possibility of retaining the prosthetic device.
Novel Antibacterial Therapeutics
New paradigms in antimicrobial therapeutic development are urgently needed to fight the rapid increase in antibiotic resistance. A variety of Gram-negative human pathogens are becoming increasingly resistant to multiple classes of antibiotics making treatment difficult. This is particularly true for hosts that suffer from chronic infections, such as those with cystic fibrosis (CF). We’ve partnered with Sarepta Therapeutics to utilize novel antisense technologies to target increasingly resistant gram-negative pathogens. Specifically, we utilize peptide-conjugated phosphorodiamidate morpholino oligomers (PPMOs) to target Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Burkholderia cepacia complex (BCC), and Pseudomonas aeruginosa. We’ve demonstrated successful inhibition of many Gram-negative bacteria by targeting essential, virulence, and antibiotic-resistant genes both in vitro and in vivo, and we continue to expand our targets.
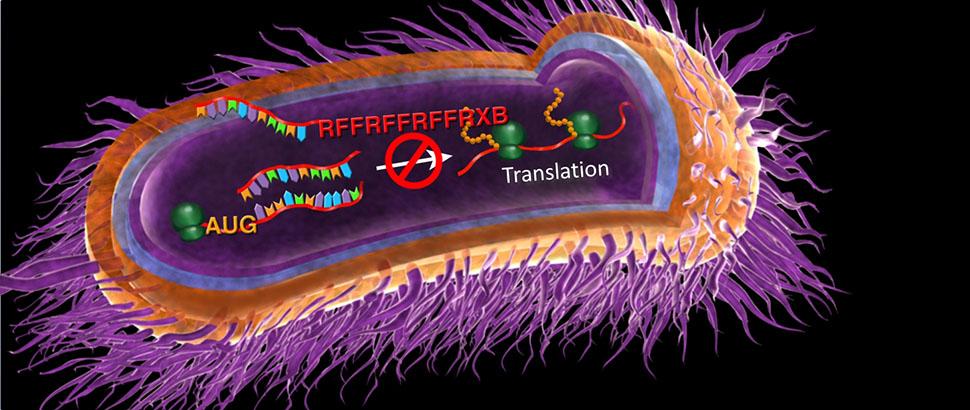 PPMOs gain entry into a bacterial cell via linked cationic-hydrophobic peptides where they can inhibit bacterial growth or virulence by inhibition of protein translation.
PPMOs gain entry into a bacterial cell via linked cationic-hydrophobic peptides where they can inhibit bacterial growth or virulence by inhibition of protein translation.