Advancing science through improvised solutions and emerging innovations to improve the delivery of medical care.
Dr. Okuda originally described the radiologically isolated syndrome in 2009, highlighting a group of individuals with incidentally identified magnetic resonance imaging features highly suspicious of autoimmune inflammatory demyelination but without symptoms associated with multiple sclerosis (MS). The “Okuda Criteria” describes the original clinical and imaging characteristics required for the appropriate classification of subjects with the pre-clinical form of MS.
The Radiologically Isolated Syndrome Consortium (RISC), a multi-center working group aimed at expanding our understanding of the early mechanisms of autoimmune inflammatory demyelination, was formed in 2009. Key founding members and collaborators include (in alphabetical order of last name):
- Orhun H. Kantarci, M.D. (Mayo Clinic, Rochester, MN, USA)
- Christine Lebrun-Frénay, M.D., Ph.D. (Côte d’Azur University, Nice, France)
- Darin T .Okuda, M.D. (The University of Texas Southwestern Medical Center, Dallas, TX, USA)
- Daniel Pelletier, M.D. (University of Southern California, Los Angeles, CA, USA)
- Aksel Siva, M.D. (Istanbul University Cerrahpasa School of Medicine, Istanbul, Türkiye)
Dr. Okuda, in collaboration with The University of Texas Southwestern Medical Center and RISC, achieved a historic milestone by successfully completing an inaugural therapeutic trial for those with RIS. In this multi-center, randomized, placebo-controlled trial, Tecfidera®, a Food and Drug Administration approved disease modifying therapy was found to prevent or delay the onset of a first neurological symptom associated with MS by over 90% in comparison to no treatment (https://pubmed.ncbi.nlm.nih.gov/36401339/). RISC was also successful in completing a second clinical trial involving Aubagio® in Europe that revealed a significant treatment on the suppression of evolution to clinical MS (https://pubmed.ncbi.nlm.nih.gov/37603328/). Prior to these randomized trials, RISC published the 5-year and 10-year risk for the development of a first clinical attack along with prognostic predictors. Other pivotal studies expanded work in pediatric and adolescent groups. The international consortium also was the first to report on people with RIS evolving to primary progressive multiple sclerosis. In 2023, modifications to the original 2009 Okuda Criteria were published to expand upon the recognition of those individuals at risk for RIS.
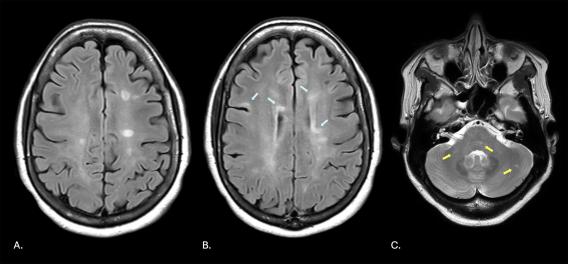
MRI of the brain demonstrating numerous regions of high signal intensity (A, in white circular shapes; B, highlighted by light blue arrows) resulting from autoimmune demyelination and within the brainstem, cerebellar peduncles, and cerebellum (C, yellow arrows).
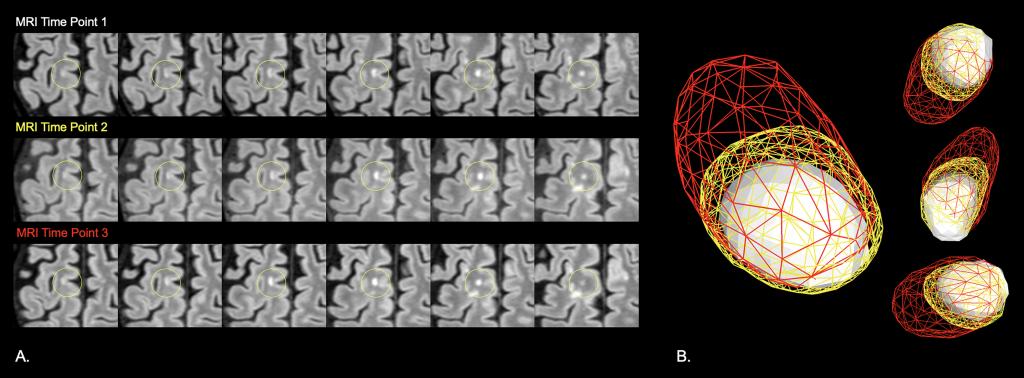
My lab developed new software capable of editing and studying lesions in their true 3D form, transforming traditional forced perspective 2D imaging constructs into engaging 3D models. In doing so, changes in 3D shape, structure, and surface characteristics could be visualized, allowing for the appreciation and capture of additional data not readily available in conventional 2D views. We studied the physiological differences within lesions and surrounding tissue based on their shapes and surface textures that lead to insights related to not only the origin of the lesions but, in the case of MS, the age of the lesion and its potential for self-repair. We have also begun studying lesion dynamics, comparing the transition of lesions over time in those with MS and those with non-specific white matter disease. Our recent data suggest that 3D morphometric and positional changes may inform on disease state and activity.
This original imaging platform that I designed has been used for exploratory outcomes in many phase 2, phase 3, and phase 4 studies within the field of MS. Because this 3D imaging software enables the study of normal anatomical structures impacted by disease, the impact of our software has already extended beyond the field of MS. We used our novel approach to evaluate a region at the dorsal medulla in people with neuromyelitis optica spectrum disorder (NMOSD) and MS and identified differences in surface topographies between groups that were highly sensitive and specific for NMOSD. Accordingly, the platform is also currently being used within a phase 4 NMOSD study aimed at identifying silent progression.
More recently, work from my lab demonstrated the dynamic properties of T2-weighted hyperintense lesions and the limitations of human visual perception in detecting such changes when comparing imaging studies side-by-side. Our reported findings highlight the value of future computerized approaches in defining disease stability, advancement, or regression.
MRI of the brain demonstrating numerous regions of high signal intensity (A, in white circular shapes; B, highlighted by light blue arrows) resulting from autoimmune demyelination and within the brainstem, cerebellar peduncles, and cerebellum (C, yellow arrows).

Magnitude of recovered new (factory sealed) disease modifying therapies used in the treatment of multiple sclerosis.
My lab developed new software capable of editing and studying lesions in their true 3D form, transforming traditional forced perspective 2D imaging constructs into engaging 3D models. In doing so, changes in 3D shape, structure, and surface characteristics could be visualized, allowing for the appreciation and capture of additional data not readily available in conventional 2D views. We studied the physiological differences within lesions and surrounding tissue based on their shapes and surface textures that lead to insights related to not only the origin of the lesions but, in the case of MS, the age of the lesion and its potential for self-repair. We have also begun studying lesion dynamics, comparing the transition of lesions over time in those with MS and those with non-specific white matter disease. Our recent data suggest that 3D morphometric and positional changes may inform on disease state and activity.
This original imaging platform that I designed has been used for exploratory outcomes in many phase 2, phase 3, and phase 4 studies within the field of MS. Because this 3D imaging software enables the study of normal anatomical structures impacted by disease, the impact of our software has already extended beyond the field of MS. We used our novel approach to evaluate a region at the dorsal medulla in people with neuromyelitis optica spectrum disorder (NMOSD) and MS and identified differences in surface topographies between groups that were highly sensitive and specific for NMOSD. Accordingly, the platform is also currently being used within a phase 4 NMOSD study aimed at identifying silent progression.
More recently, work from my lab demonstrated the dynamic properties of T2-weighted hyperintense lesions and the limitations of human visual perception in detecting such changes when comparing imaging studies side-by-side. Our reported findings highlight the value of future computerized approaches in defining disease stability, advancement, or regression.
Our group was the first to transform grayscale magnetic resonance imaging (MRI) data to full color using generative artificial intelligence (AI). AI platforms involved in color transformation are able to detect subtle variations in brightness, grouping them into thousands of segmentation channels. Feature vectors may then be applied for both segmentation and color assignment.
Color provides hue, saturation, and brightness while conventional magnetic resonance imaging data only provide brightness (intensity).The application of color in neuroimaging may provide greater contextual clarity in comparison to current grayscale images for those without reduced sensitivity to red, green, and blue light. With advancing artificial intelligence methods and capabilities along with the additional data that color provides in comparison to grayscale, new insights into the biology of disease may be recognized. Modifying what we measure in people with chronic conditions and how we present the data may be of greater value than conventional approaches typically used in the study, education, and care of people with MS and other neurological conditions.
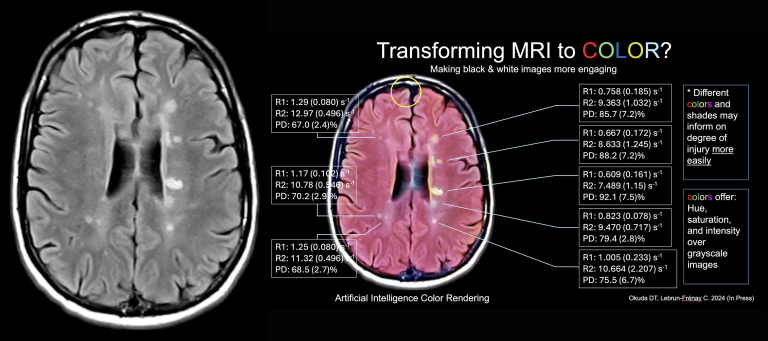
Transforming grayscale MRI data (left) into full color (right) using generative artificial intelligence. Supporting quantitative MRI data are provided that support the color renderings created by the platform.
Can the brain elicit different forms of music based on structural anomalies present? We believe so. Using generative artificial intelligence and a variety of 3-dimensional measures, we were able to generate music from the brain of a person with multiple sclerosis. Below is an example of generated music before and after treatment.