Cell Mechanics and Corneal Wound Healing
Located in the Department of Ophthalmology, the Petroll Lab applies engineering approaches and design principles to the investigation of fundamental clinical and biological problems in ophthalmology, while providing training to graduate students, medical students, and postdocs.
Research
The main focus of the Petroll Lab’s research is cell mechanics and tissue engineering, whereby multidimensional time-lapse imaging is used to investigate how the mechanical behavior of corneal fibroblasts is regulated by both biochemical and biophysical stimuli. Our lab also has a longstanding interest in the development and application of in vivo confocal microscopy and in situ multiphoton imaging, which allow quantitative 3-D assessment of cell differentiation and extracellular matrix organization during corneal wound healing in response to clinical procedures such as refractive surgery or UV cross-linking.
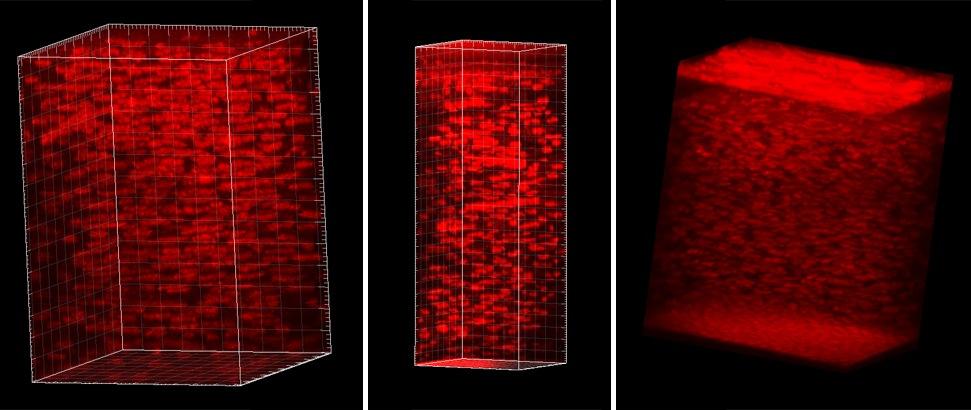 We develop and apply in vivo confocal microscopy and in situ multiphoton imaging techniques to assess the cellular responses to corneal injury, disease and refractive surgical procedures.
We develop and apply in vivo confocal microscopy and in situ multiphoton imaging techniques to assess the cellular responses to corneal injury, disease and refractive surgical procedures. We use multidimensional time-lapse imaging to investigate how the behavior of corneal fibroblasts is regulated by biochemical and biophysical stimuli encountered during wound healing.

Overview
The organization of extracellular matrices by cells through the exertion of mechanical forces drives fundamental processes such as developmental morphogenesis, wound healing, and the organization of bioengineered tissues. Historically, our ability to investigate cell mechanical behavior has been limited by the technical challenges associated with measuring the sub-cellular origins of cellular force generation and local matrix patterning in a 3-D environment. Thus, our understanding of these fundamental processes is limited, especially in ocular tissues. Over the last several years, our research team has addressed these challenges through the development of new experimental models, use of emerging imaging technologies, and the application of quantitative analysis techniques.
Experimental Models
Our in vitro approaches include: 1) models for assessing how specific growth factors expressed following injury or surgery alter the mechanical differentiation of quiescent corneal keratocytes within 3-D collagen matrices, 2) novel approaches for assessing the response of fibroblasts to dynamic changes in ECM mechanical properties (as might be observed during development or wound healing), 3) 3-D constructs for assessing the mechanical interplay between fibroblast migration, sub-cellular force generation, and ECM patterning, and 4) engineered substrates that allow investigation of the impact of ECM protein composition, topography, and elasticity on cell differentiation and mechanical behavior.
Together, these experimental models are providing unique insights into the underlying biochemical and biomechanical signaling mechanisms controlling corneal fibroblast migration, contraction, and matrix reorganization. This is fundamental information which can not be obtained using standard 2-D culture models, and may eventually lead to improved strategies for modulating cell mechanical activity during wound healing, and for designing artificial matrices and directing cell behavior during corneal tissue engineering.
The cornea is an optically clear tissue that forms the front surface of the eye, and accounts for nearly two-thirds of its refractive power. The corneal stroma, which makes up 90% of corneal thickness, is a highly organized structure consisting of collagen lamellae with specific packing and spacing that is critical to maintenance of corneal transparency. Corneal stromal cells (keratocytes) reside between the collagen lamellae, and are responsible for secreting extracellular matrix (ECM) components required to maintain normal corneal structure and function (i.e. transparency). Because it is exposed, the cornea is susceptible to infection, physical and chemical injuries; it is also the target of many vision correction procedures.
Stromal keratocytes play a central role in mediating the corneal response to lacerating injury, chemical injuries or surgical procedures. During wound healing, quiescent corneal keratocytes surrounding the area of injury generally become activated, and transform into a fibroblastic repair phenotype. In certain wound types, fibroblasts further differentiate into myofibroblasts, which generate stronger forces and synthesize a disorganized fibrotic ECM. Cellular force generation and fibrosis can alter corneal shape and/or reduce corneal transparency, thereby reducing visual acuity.
In addition to fibrosis which develops on top of the wound bed, most vision correction procedures induce keratocyte death beneath the laser-treated area. Stromal cell death can also be induced by toxic injury, as well as UV cross-linking (CXL) of the cornea in keratoconus patients. Ideally, repopulation following these insults should occur via intra-stromal migration of keratocytes from the surrounding stromal tissue, without generation of strong contractile forces that could disrupt the collagen architecture and lead to vision loss.
Our lab develops and applies high resolution imaging and image processing approaches to better understand and potentially modulate these healing responses. Specifically, we use in vivo confocal microscopy to assess stromal keratocyte differentation, patterning and activation in response corneal injury, surgery, or disease. We also use 3-D confocal and multiphoton imaging of corneal tissue ex vivo to assess the expression and localization of specific proteins associated with wound healing (using fluorescent labeling) as well as changes in extracellular matrix organization (using second harmonic generation imaging).1,2
References
-
Petroll WM, Kivanany PB, Hagenasr D, Graham EK. Corneal Fibroblast Migration Patterns During Intrastromal Wound Healing Correlate With ECM Structure and Alignment. Invest Ophthalmol Vis Sci. 2015 Nov;56(12):7352-61.
-
Kivanany PB, Grose KC, Tippani M, Su S, Petroll WM. Assessment of Corneal Stromal Remodeling and Regeneration after Photorefractive Keratectomy. Sci Rep. 2018 Aug 22;8(1):12580.
Video: About the Petroll Lab
Meet the Principal Investigator

W. Matthew Petroll, Ph.D.
Dr. Petroll is a Professor of Ophthalmology and Biomedical Engineering at UT Southwestern Medical Center. He received his B.S. degree in Biomedical Engineering from Duke University in 1984, and his Ph.D. in Biomedical Engineering from the University of Virginia in 1989. After two years at Georgetown University in the lab of Drs. James Jester and Dwight Cavanagh, he joined the faculty at UT Southwestern in 1991. He was promoted to professor in 2005, and became Chair of the Graduate Program in Biomedical Engineering in 2012.
Dr. Petroll’s laboratory applies engineering approaches and design principles to the investigation of fundamental clinical and biological problems in ophthalmology, while providing training to graduate students, medical students, and post-docs. The main focus of Dr. Petroll’s research is cell mechanics and tissue engineering, in which multidimensional time-lapse imaging is used to investigate how the mechanical behavior of corneal fibroblasts is regulated by both biochemical and biophysical stimuli. He also has a longstanding interest in the development and application of in vivo confocal microscopy, which allows quantitative 3-D imaging of the cornea and has been used in numerous research and clinical studies on corneal wound healing, toxicity, development and infection.
Publications
Single cell RNA-seq characterization of non-fibrotic stromal wound repopulation in the rabbit
Borner K, Yu Z, Xing C, Petroll WM 2026 Mar Experimental Eye Research 264Effect of Decorin and Aligned Collagen Fibril Topography on TGF-β1 Activation of Corneal Keratocytes
Tjahjono NS, Subramanian D, Shihabeddin TZ, Hicks HD, Varner VD, Petroll WM, Schmidtke DW 2025 Mar Bioengineering 12The Role of Vimentin in Human Corneal Fibroblast Spreading and Myofibroblast Transformation
Miron-Mendoza M, Poole K, DiCesare S, Nakahara E, Bhatt MP, Hulleman JD, Petroll WM 2024 Jul Cells 13The impact of UV cross-linking on corneal stromal cell migration, differentiation and patterning
Petroll WM, Miron-Mendoza M, Sunkara Y, Ikebe HR, Sripathi NR, Hassaniardekani H 2023 Aug Experimental Eye Research 233Sutureless Conjunctiva-Sparing Posterior Ptosis Repair Surgery: A Novel Technique
Mancini R, Forouzan P, Keenum ZG, Tenzel PA, Petroll WM 2023 Jul American journal of ophthalmology 251 77-89Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces
Lam KH, Shihabeddin TZ, Awkal JA, Najjar AM, Miron-Mendoza M, Maruri DP, Varner VD, Petroll WM, Schmidtke DW 2023 Apr Journal of Functional Biomaterials 14Infectious keratitis after corneal crosslinking: systematic review
Murchison CE, Petroll WM, Robertson DM 2021 Aug Journal of Cataract and Refractive Surgery 47 1075-1080ECM Stiffness Controls the Activation and Contractility of Corneal Keratocytes in Response to TGF-β1
Maruri DP, Miron-Mendoza M, Kivanany PB, Hack JM, Schmidtke DW, Petroll WM, Varner VD 2020 Nov Biophysical journal 119 1865-1877Fibulin-3 knockout mice demonstrate corneal dysfunction but maintain normal retinal integrity
Daniel S, Renwick M, Chau VQ, Datta S, Maddineni P, Zode G, Wade EM, Robertson SP, Petroll WM, Hulleman JD 2020 Nov Journal of Molecular Medicine 98 1639-1656Outcomes of resident-performed laser-assisted vs traditional phacoemulsification
Hansen B, Blomquist PH, Ririe P, Pouly S, Nguyen C, Petroll WM, McCulley JP 2020 Sep Journal of Cataract and Refractive Surgery 46 1273-1277Assessment of Corneal Stromal Remodeling and Regeneration after Photorefractive Keratectomy
Kivanany PB, Grose KC, Tippani M, Su S, Petroll WM 2018 Dec Scientific reports 8Fibroblast-fibronectin patterning and network formation in 3D fibrin matrices
Miron-Mendoza M, Graham E, Manohar S, Petroll WM 2017 Dec Matrix Biology 64 69-80Second harmonic generation imaging of corneal stroma after infection by Pseudomonas aeruginosa
Robertson DM, Rogers NA, Petroll WM, Zhu M 2017 Apr Scientific reports 7Impact of donor age on corneal endothelium-descemet membrane layer scroll formation
Bennett A, Mahmoud S, Drury D, Cavanagh HD, McCulley JP, Petroll WM, Mootha V 2015 Jul Eye and Contact Lens 41 236-239Effect of HDAC inhibitors on corneal keratocyte mechanical phenotypes in 3-D collagen matrices
Koppaka V, Lakshman N, Matthew Petroll W 2015 Apr Molecular Vision 21 502-514Mechanical interactions and crosstalk between corneal keratocytes and the extracellular matrix
Petroll WM, Miron-Mendoza M 2015 Apr Experimental Eye Research 133 49-57TCF4 Triplet Repeat Expansion and Nuclear RNA Foci in Fuchs' Endothelial Corneal Dystrophy
Mootha V, Hussain I, Cunnusamy K, Graham E, Gong X, Neelam S, Xing C, Kittler R, Petroll WM 2015 Mar Investigative ophthalmology & visual science 56 2003-2011Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis
Ding Z, Joy M, Bhargava R, Gunsaulus M, Lakshman N, Miron-Mendoza M, Petroll M, Condeelis J, Wells A, Roy P 2014 Apr Oncogene 33 2065-2074MMP regulation of corneal keratocyte motility and mechanics in 3-D collagen matrices
Zhou C, Petroll WM 2014 Apr Experimental Eye Research 121 147-160Techniques for assessing 3-D cell-matrix mechanical interactions in vitro and in vivo
Miron-Mendoza M, Koppaka V, Zhou C, Petroll WM 2013 Oct Experimental Cell Research 319 2470-2480Quantitative 3-dimensional corneal imaging in vivo using a modified hrt-rcm confocal microscope
Petroll WM, Weaver M, Vaidya S, McCulley JP, Cavanagh HD 2013 Apr Cornea 32 e36-e43Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices
Lakshman N, Matthew Petroll W 2012 Mar Investigative Ophthalmology and Visual Science 53 1077-1086A reconstituted telomerase-immortalized human corneal epithelium in vivo: A pilot study
Robertson DM, Kalangara JP, Baucom RB, Petroll WM, Cavanagh HD 2011 Aug Current Eye Research 36 706-712Cell motility and mechanics in three-dimensional collagen matrices
Grinnell F, Petroll WM 2010 Nov Annual Review of Cell and Developmental Biology 26 335-361Castroviejo lecture 2009: 40 years in search of the perfect contact lens
Cavanagh HD, Robertson DM, Petroll WM, Jester JV 2010 Oct Cornea 29 1075-1085Inducible macropinocytosis of hyaluronan in B16-F10 melanoma cells
Greyner HJ, Wiraszka T, Zhang LS, Petroll WM, Mummert ME 2010 Jul Matrix Biology 29 503-510Rho kinase regulation of fibroblast migratory mechanics in fibrillar collagen matrices
Zhou C, Petroll WM 2010 Mar Cellular and Molecular Bioengineering 3 76-83Development of a hyaluronan bioconjugate for the topical treatment of melanoma
Zhang LS, Petroll WM, Greyner HJ, Mummert ME 2009 Jul Journal of Dermatological Science 55 56-59Outcomes of PermaVision Intracorneal Implants for the Correction of Hyperopia
Verity S, McCulley JP, Bowman RW, Cavanagh HD, Petroll WM 2009 Jun American journal of ophthalmology 147 973-977Intraocular pressure reduction in the untreated fellow eye after selective laser trabeculoplasty
Rhodes KM, Weinstein R, Saltzmann RM, Aggarwal N, Kooner KS, Petroll WM, Whitson JT 2009 Mar Current Medical Research and Opinion 25 787-796Intraocular pressure reduction in the untreated fellow eye after selective laser trabeculoplasty
Rhodes KM, Weinstein R, Saltzmann RM, Aggarwal N, Kooner KS, Petroll WM, Whitson JT 2009 Mar Current Medical Research and Opinion 25 787-796Technique article: An experimental model for assessing fibroblast migration in 3-D collagen matrices
Karamichos D, Lakshman N, Petroll WM 2009 Jan Cell Motility and the Cytoskeleton 66 1-9Localized application of mechanical and biochemical stimuli in 3-D culture
Petroll WM, Ma L 2008 Oct Developmental Dynamics 237 2726-2736Analysis of the pattern of subcellular force generation by corneal fibroblasts after Rho activation
Petroll WM, Ma L, Ly L, Vishwanath M 2008 Jan Eye and Contact Lens 34 65-70Slit-lamp, confocal, and light microscopic findings of corneal siderosis
Witherspoon SR, Hogan RN, Petroll WM, Mootha V 2007 Dec Cornea 26 1270-1272Insulin-like Growth Factor Binding Protein-3 expression in the human corneal epithelium
Robertson DM, Ho SI, Hansen BS, Petroll WM, Cavanagh HD 2007 Oct Experimental Eye Research 85 492-501Microtubule regulation of corneal fibroblast morphology and mechanical activity in 3-D culture
Kim A, Matthew Petroll W 2007 Oct Experimental Eye Research 85 546-556Postnatal corneal transparency, keratocyte cell cycle exit and expression of ALDH1A1
Jester JV, Lee YG, Huang J, Houston J, Adams B, Cavanagh HD, Petroll WM 2007 Sep Investigative Ophthalmology and Visual Science 48 4061-4069Rho plays a central role in regulating local cell-matrix mechanical interactions in 3D culture
Lakshman N, Kim A, Bayless KJ, Davis GE, Petroll WM 2007 Jun Cell Motility and the Cytoskeleton 64 434-445Current concepts: Contact lens related Pseudomonas keratitis
Robertson DM, Petroll WM, Jester JV, Cavanagh HD 2007 May Contact Lens and Anterior Eye 30 94-107Prospective evaluation of PermaVision intracorneal implants using in vivo confocal microscopy
Lindsey SS, McCulley JP, Cavanagh HD, Verity S, Bowman RW, Petroll WM 2007 Apr Journal of Refractive Surgery 23 410-413Local thermal injury elicits immediate dynamic behavioural responses by corneal Langerhans cells
Ward BR, Jester JV, Nishibu A, Vishwanath M, Shalhevet D, Kumamoto T, Petroll WM, Cavanagh HD, Takashima A 2007 Apr Immunology 120 556-572Dynamic assessment of cell-matrix mechanical interactions in three-dimensional culture.
Petroll WM 2007 Methods in molecular biology (Clifton, N.J.) 370 67-82Quantitative assessment of local collagen matrix remodeling in 3-D Culture: The role of Rho kinase
Kim A, Lakshman N, Petroll WM 2006 Nov Experimental Cell Research 312 3683-3692Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals
Morishige N, Petroll WM, Nishida T, Kenney MC, Jester JV 2006 Nov Journal of Cataract and Refractive Surgery 32 1784-1791Confocal assessment of the effects of fourth-generation fluoroquinolones on the cornea
Ly LT, Cavanagh HD, Petroll WM 2006 Jul Eye and Contact Lens 32 161-165Tandem Scanning Confocal Corneal Microscopy in the Diagnosis of Suspected Acanthamoeba Keratitis
Parmar DN, Awwad ST, Petroll WM, Bowman RW, McCulley JP, Cavanagh HD 2006 Apr Ophthalmology 113 538-547Bcl-2 and Bax regulation of corneal homeostasis in genetically altered mice
Robertson DM, Ladage PM, Yamamoto N, Jester JV, Petroll WM, Cavanagh HD 2006 Jan Eye and Contact Lens 32 3-7In-vivo microvasculature visualization using hyperspectral imaging
Shah BB, Cavanagh HD, Petroll WM, Kothare AD, Gundabhat P, Behbehani K, Zuzak KJ 2006Real-time high spatial resolution, in vivo corneal imaging: Current successes and future needs
Cavanagh HD, Petroll WM 2006Central corneal thickness in patients with congenital aniridia
Whitson JT, Liang C, Godfrey DG, Petroll WM, Cavanagh HD, Patel D, Fellman RL, Starita RJ 2005 Sep Eye and Contact Lens 31 221-224Corneal fibroblasts respond rapidly to changes in local mechanical stress
Petroll WM, Vishwanath M, Ma L 2004 Oct Investigative Ophthalmology and Visual Science 45 3466-3474Pseudomonas aeruginosa corneal binding after 24-hour orthokeratology lens wear
Ladage PM, Yamamoto N, Robertson DM, Jester JV, Petroll WM, Cavanagh HD 2004 Jul Eye and Contact Lens 30 173-178Four-dimensional multiphoton confocal microscopy: The new frontier in cellular imaging
Jester JV, Ward BR, Takashima A, Gatlin J, Garcia JV, Cavanagh HD, Petroll WM 2004 Jan Ocular Surface 2 10-20Effect of ophthalmic viscosurgical devices on lens epithelial cells: A morphological study
Budo C, Goffinet G, Bellotto D, Petroll WM 2003 Dec Journal of Cataract and Refractive Surgery 29 2411-2418Modulation of Corneal Fibroblast Contractility within Fibrillar Collagen Matrices
Vishwanath M, Ma L, Otey CA, Jester JV, Petroll WM 2003 Nov Investigative Ophthalmology and Visual Science 44 4724-4735Direct, dynamic assessment of cell-matrix interactions inside fibrillar collagen lattices
Petroll WM, Ma L 2003 Aug Cell Motility and the Cytoskeleton 55 254-264In vivo fluorescent labeling of corneal wound healing fibroblasts
Gatlin J, Melkus MW, Padgett A, Petroll WM, Cavanagh HD, Garcia JV, Jester JV 2003 Mar Experimental Eye Research 76 361-371Neonatal corneal stromal development in the normal and lumican-deficient mouse
Song J, Lee YG, Houston J, Petroll WM, Chakravarti S, Cavanagh HD, Jester JV 2003 Feb Investigative Ophthalmology and Visual Science 44 548-557Can postlens tear thickness be measured using three-dimensional in vivo confocal microscopy?
Petroll WM, Kovoor T, Ladage PM, Cavanagh HD, Jester JV, Robertson DM 2003 Jan Eye & contact lens 29 S110-114; discussion S115-118, S192-194Role of oxygen in corneal epithelial homeostasis during extended contact lens wear.
Ladage PM, Jester JV, Petroll WM, Bergmanson JP, Cavanagh HD 2003 Jan Eye & contact lens 29 S2-6; discussion S26-29, S192-194Spherical Indentations of Human and Rabbit Corneal Epithelium Following Extended Contact Lens Wear
Ladage PM, Petroll WM, Jester JV, Fisher S, Bergmanson JP, Cavanagh HD 2002 Oct CLAO Journal 28 177-180Corneal epithelial homeostasis following daily and overnight contact lens wear
Ladage PM, Yamamoto K, Li L, Ren DH, Petroll WM, Jester JV, Cavanagh HD 2002 Contact Lens and Anterior Eye 25 11-21Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4
Khatri S, Lass JH, Heinzel FP, Petroll WM, Gomez J, Diaconu E, Kalsow CM, Pearlman E 2002 Investigative Ophthalmology and Visual Science 43 2278-2284A prototype two-detector confocal microscope for in vivo corneal imaging
Petroll WM, Yu A, Li J, Jester JV, Cavanagh HD, Black T 2002 Scanning 24 163-170Surgically induced astigmatism after hyperopic and myopic photorefractive keratectomy
Yi DH, Petroll WM, Bowman RW, McCulley JP, Cavanagh HD 2001 Journal of Cataract and Refractive Surgery 27 396-403Organization of junctional proteins in proliferating cat corneal endothelium during wound healing
Petroll WM, Ma L, Jester JV, Cavanagh HD, Bean J 2001 Cornea 20 73-80Proliferation rate of rabbit corneal epithelium during overnight rigid contact lens wear
Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Petroll WM, Bergmanson JP, Cavanagh HD 2001 Investigative Ophthalmology and Visual Science 42 2804-2812Pathology of ocular irritation with bleaching agents in the rabbit low-volume eye test
Maurer JK, Molai A, Parker RD, Li L, Carr GJ, Petroll WM, Cavanagh HD, Jester JV 2001 Toxicologic Pathology 29 308-319Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves
Linna TU, Vesaluoma MH, Pérez-Santonja JJ, Petroll WM, Alió JL, Tervo TM 2000 Feb Investigative Ophthalmology and Visual Science 41 393-397Effect of eyelid closure on the epithelial proliferation rate of the rabbit cornea
Ladage PM, Yamamoto K, Li L, Petroll M, Jester JV, Bergmanson JP, Cavanagh HD 2000 Optometry and Vision Science 77 173Quantitative characterization of acid- and alkali-induced corneal injury in the low-volume eye test
Jester JV, Molai A, Petroll WM, Parker RD, Carr GJ, Cavanagh HD, Maurer JK 2000 Toxicologic Pathology 28 668-678Autosomal recessive cornea plana: In vivo corneal morphology and corneal sensitivity
Vesaluoma MH, Sankila EM, Gallar J, Müller LJ, Petroll WM, Moilanen JA, Forsius H, Tervo TM 2000 Investigative Ophthalmology and Visual Science 41 2120-2126Comparison of contractile force generated by isolated and paired fibroblasts in vitro
Mariappan MR, Petroll WM, Cavanagh HD, Jester JV 1999 DecChanges in corneal endothelial apical junctional protein organization after corneal cold storage
Hsu JK, Cavanagh HD, Jester JV, Ma L, Petroll WM 1999 Nov Cornea 18 712-720Corneal stromal wound healing in refractive surgery: The role of myofibroblasts
Jester JV, Petroll WM, Cavanagh HD 1999 Jul Progress in Retinal and Eye Research 18 311-356Effect of Cell Migration on the Maintenance of Tension on a Collagen Matrix
Roy P, Petroll WM, Chuong CJ, Cavanagh HD, Jester JV 1999 Annals of biomedical engineering 27 721-730The cellular basis of corneal transparency: Evidence for 'corneal crystallins'
Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J 1999 Journal of cell science 112 613-622Area and depth of surfactant-induced corneal injury predicts extent of subsequent ocular responses
Jester JV, Petroll WM, Bean J, Parker RD, Carr GJ, Cavanagh HD, Maurer JK 1998 Dec Investigative Ophthalmology and Visual Science 39 2610-2625Area and depth of surfactant-induced corneal injury correlates with cell death
Jester JV, Li HF, Petroll WM, Parker RD, Cavanagh HD, Carr GJ, Smith B, Maurer JK 1998 May Investigative Ophthalmology and Visual Science 39 922-936Confocal microscopic characterization of wound repair after photorefractive keratectomy
Møller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV 1998 Mar Investigative Ophthalmology and Visual Science 39 487-501Corneal haze development after PRK is regulated by volume of stromal tissue removal
Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV 1998 Cornea 17 627-639Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF(β) in the rabbit
Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD 1997 Mar Cornea 16 177-187Characterization of SV40-transfected cell strains from rabbit keratocytes
Barry-Lane PA, Wilson SE, Cavanagh HD, Petroll WM, Jester JV 1997 Jan Cornea 16 72-78Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF)
Li HF, Petroll WM, Møller-Pedersen T, Maurer JK, Cavanagh HD, Jester JV 1997 Current Eye Research 16 214-221Area and depth of surfactant-induced corneal injury correlates with cell death
Li TF, Maurer JK, Parker RD, Petroll WM, Cavanagh HD, Jester JV 1997 Investigative Ophthalmology and Visual Science 38 S399Tgfβ mediated corneal myofibroblast transformation is dependent on tyrosine phosphorylation
Jester JV, Huang J, Kao WW, Petroll WM, Cavanagh HD 1997 Investigative Ophthalmology and Visual Science 38 S502Does hypoxia alone increase the bacterial adherence to exfoliated corneal epithelial cells in man?
Ren H, Ho-Fan J, Petroll WM, Jester JV, Cavanagh HD 1997 Investigative Ophthalmology and Visual Science 38 S139In vivo confocal microscopy: a new possibility to confirm the diagnosis of Borrelia keratitis?
Linna T, Mikkilä H, Karma A, Seppälä I, Petroll WM, Tervo T 1996 Nov Cornea 15 639-640TGFβ1 modulated myofibroblast transformation via matrix interaction: Role in wound contraction
Jester JV, Petroll WM, Huang J, Roy P, Barry-Lane PA, Cavanagh HD 1996 Feb Investigative Ophthalmology and Visual Science 37 S219Measurement of Surgically Induced Corneal Deformations Using Three-Dimensional Confocal Microscopy
Petroll WM, Roy P, Chuong CJ, Hall B, Cavanagh HD, Jester JV 1996 Cornea 15 154-164Expression of α-smooth muscle (α-SM) actin during corneal stromal wound healing
Jester JV, Petroll WM, Barry PA, Cavanagh HD 1995 Investigative Ophthalmology and Visual Science 36 809-819Temporal, 3-dimensional, cellular anatomy of corneal wound tissue
Jester JV, Petroll WM, Barry PA, Cavanagh HD 1995 Journal of Anatomy 186 301-311Quantitative assessment of anteroposterior keratocyte density in the normal rabbit cornea
Petroll WM, Boettcher K, Barry P, Cavanagh D, Jester JV 1995 Cornea 14 3-9Confocal microscopy: Uses in measurement of cellular structure and function
Cavanagh HD, Petroll WM, Jester JV 1995 Progress in Retinal and Eye Research 14 527-565Corneal keratocytes: In situ and in vitro organization of cytoskeletal contractile proteins
Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD 1994 Jan Investigative Ophthalmology and Visual Science 35 730-743Three and four dimensional scanning microscopy: Introduction
Cavanagh HD, Petroll WM, Jester JV 1994 Scanning 16 129Laser and tandem scanning confocal microscopic studies of rabbit corneal wound healing
Ichijima H, Jester JV, Petroll WM, Cavanagh HD 1994 Scanning 16 263-268In vivo confocal imaging: General principles and applications
Petroll WM, Jester JV, Cavanagh HD 1994 Scanning 16 131-149In vivo osmotic pertubation of intercellular fluid channels in the rabbit corneal endothelium
Andrews PM, Jester JV, Petroll WM, Barry PA, Ichijima H, Cavanagh HD 1994 Cornea 13 253-258Three‐dimensional imaging of corneal cells using in vivo confocal microscopy
Petroll WM, Cavanagh HD, Jester JV 1993 Jun Journal of Microscopy 170 213-219Quantitative analysis of stress fiber orientation during corneal wound contraction
Petroll WM, Cavanagh HD, Barry P, Andrews P, Jester JV 1993 Feb Journal of cell science 104 353-363Ocular water evaporation and the dry eye: A new measuring device
Mathers WD, Binarao G, Petroll M 1993 Jan Cornea 12 335-340The application of confocal microscopy to the study of living systems
Cavanagh HD, Petroll WM, Jester JV 1993 Jan Neuroscience and Biobehavioral Reviews 17 483-498Clinical and Diagnostic Use of In Vivo Confocal Microscopy in Patients with Corneal Disease
Cavanagh HD, Petroll WM, Alizadeh H, He Y, McCulley JP, Jester JV 1993 Ophthalmology 100 1444-1454In vivo confocal microscopic studies of endothelial wound healing in rabbit cornea
Ichijima H, Petroll WM, Jester JV, Barry PA, Andrews PM, Dai M, Cavanagh HD 1993 Cornea 12 369-378The application of confocal microscopy to the study of living systems
Cavanagh HD, Petroll WM, Jester JV 1993 Neuroscience and Biobehavioral Reviews 17 477-482In vivo confocal microscopy in clinical dental research: An initial appraisal
Watson TF, Petroll WM, Cavanagh HD, Jester JV 1992 Dec Journal of Dentistry 20 352-358Morphologic effects of contact lens wear on the corneal surface
Mathers WD, Sachdev MS, Petroll M, Lemp MA 1992 Jan CLAO Journal 18 49-52Radial keratotomy: II. Role of the myofibroblast in corneal wound contraction
Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV 1992 Investigative Ophthalmology and Visual Science 33 3271-3282Radial keratotomy: III. Relationship between wound gape and corneal curvature in primate eyes
Petroll WM, New K, Sachdev M, Cavanagh HD, Jester JV 1992 Investigative Ophthalmology and Visual Science 33 3283-3291Confocal microscopic studies of living rabbit cornea treated with benzalkonium chloride
Ichijima H, Petroll WM, Jester JV, Cavanagh HD 1992 Cornea 11 221-225Tandem Scanning Confocal Microscopy (TSCM) of normal and ischemic living kidneys
Andrews PM, Petroll WM, Cavanagh HD, Jester JV 1991 May American Journal of Anatomy 191 95-102Relationships between abdominal and diaphragmatic volume displacements
Knight H, Petroll WM, Rochester DF 1991 Jan Journal of applied physiology 71 565-572In vivo imaging of human teeth and skin using real‐time confocal microscopy
New KC, Petroll WM, Boyde A, Martin L, Corcuff P, Leveque JL, Lemp MA, Cavanagh HD, Jester JV 1991 Scanning 13 369-372In vitro artifacts vs in vivo reality with confocal microscopy
Jester JV, Petroll WM, Garana R, Lemp MA, Cavanagh HD 1991 Scanning 13 I91-I92Meibomian gland morphology and tear osmolarity: Changes with accutane therapy
Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV 1991 Cornea 10 286-290In vivo 3-dimensional reconstruction of corneal fibroblasts using confocal microscopy
Petroll WM, Hill J, Cavanagh HD, Jester JV 1991 Scanning 13 I92-I93Fluorescent staining of intact, unsectioned corneal tissue for analysis by confocal microscopy
Barry PA, Petroll WM, Garana RM, Cavanagh HD, Jester JV 1991 Scanning 13 I112A model approach to assess diaphragmatic volume displacement
Petroll WM, Knight H, Rochester DF 1990 Journal of applied physiology 69 2175-2182Effect of lower rib cage expansion and diaphragm shortening on the zone of apposition
Petroll WM, Knight H, Rochester DF 1990 Journal of applied physiology 68 484-488Videofluoroscopic assessment of muscle fiber shortening in the in situ canine diaphragm
Knight H, Petroll WM, Adams JM, Shaffer HA, Rochester DF 1990 Journal of applied physiology 68 2200-2207Contact Petroll Lab
We want to hear from students and postdoctoral candidates who are interested in joining our lab. Please contact us for more information.
W. Matthew Petroll, Ph.D.
Professor of Ophthalmology Vice Chair, Research
Chair, Biomedical Engineering Graduate Program
Email
Mailing Address
Department of Ophthalmology
UT Southwestern Medical Center
5323 Harry Hines Blvd.
Dallas, TX 75390-9057
 l. to r.: Kate Borner (BME grad student), Miguel Miron Mendoza, Ph.D. (Sr. Research Scientist), W. Matthew Petroll, Ph.D., Hajar Hassaniardekani, Ph.D. (Postdoc), and Kara Poole (BME grad student)
l. to r.: Kate Borner (BME grad student), Miguel Miron Mendoza, Ph.D. (Sr. Research Scientist), W. Matthew Petroll, Ph.D., Hajar Hassaniardekani, Ph.D. (Postdoc), and Kara Poole (BME grad student)