Tau protein, which normally functions to stabilize the physical framework of cells, sometimes take on a shape that creates tangles in human brains that are linked to neurodegenerative diseases called tauopathies.
We want to understand how the inert form transforms into the pathological shape. A key tool in our research is a technique called cross-linking mass spectrometry (XL-MS), which creates chemical bonds among adjacent portions of the protein to reveal architectural information.
Recently we purified and characterized a conformation of tau monomer that serves as a “seed” that drives aggregation, which we named Ms. Using XL-MS, we found that Ms's shape exposes a portion of the protein that is normally hidden within inert tau monomer (Mi).
We are now studying how different conformations of tau are related to different tauopathies – whether, for instance, tau aggregates in Alzheimer’s disease differ from those in Parkinson’s disease. A clearer understanding of the shapes these conformations adopt will have important implications for development of better diagnostic and therapeutic strategies.
References:
Mirbaha H, et al (2018). Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife:e36584.
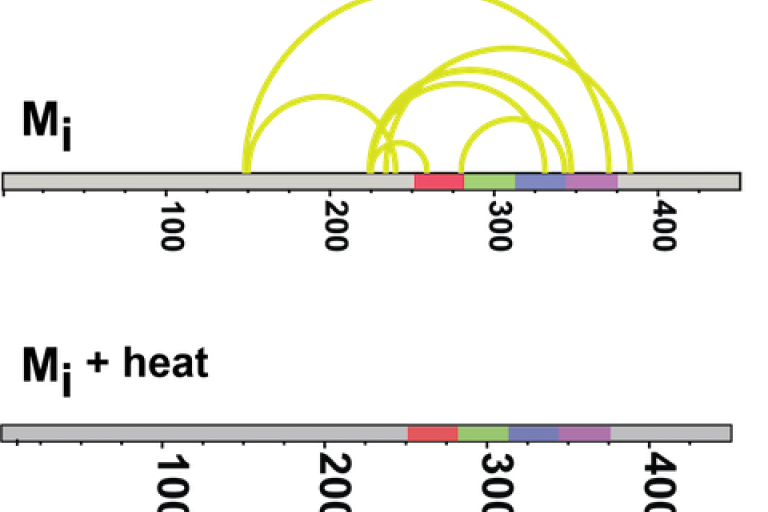
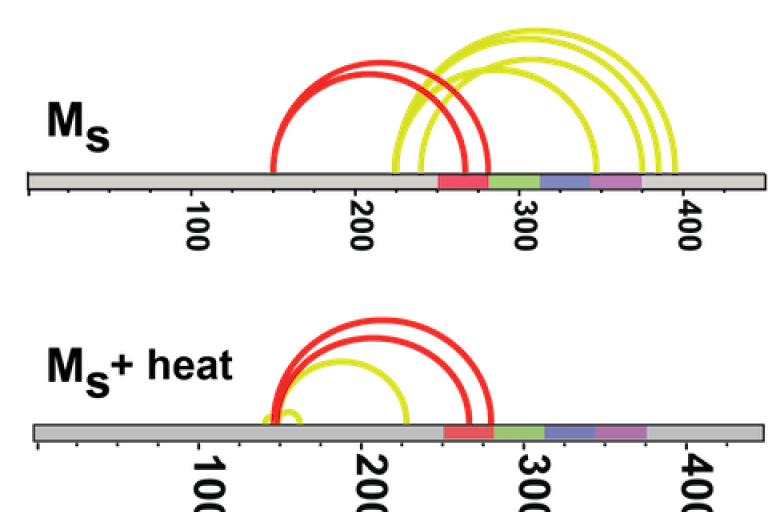
About the Image: Crosslinking studies on inert tau monomer (Mi) and tau seeding monomer (Ms) reveal different crosslink patterns. Ms crosslinks are also more stable when heated.