About Our Research
Oocytes are life's time capsules. They can hibernate for days to decades before they experience a dramatic awakening, called activation. Once fertilized, these oocytes reprogram themselves to become a rapidly dividing embryo. How does an oocyte organize its cytoplasm to survive hibernation and protect itself against aging? How is the oocyte reorganized to promote rapid, mitotic divisions? What are the safeguards to prevent and correct errors in this crucial reprogramming?
Our mission is to understand the design principles of cytoplasmic reorganization during oocyte storage, activation, and fertilization.
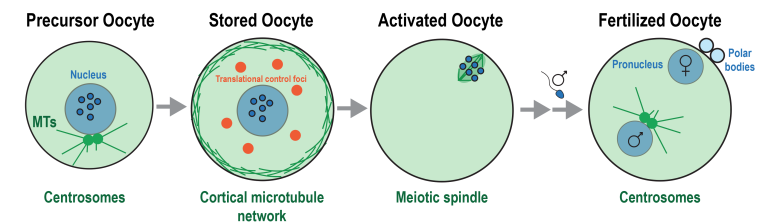
Female mammals must store their oocytes over years to decades before they are ovulated. A decrease in oocyte quality over time is a major factor in age-related infertility. We study how the oocyte cytoplasm is reorganized to shut down cellular metabolism and protect its precious contents to survive long-term hibernation.
Oocytes and embryos compartmentalize their cytoplasm by building supramolecular assemblies of proteins and nucleic acids, termed "biomolecular condensates". We want to understand how these condensates control protein translation, metabolism, and cytoskeletal organization during the oocyte-to-embryo transition.
After fertilization, the oocyte switches to mitotic cell divisions orchestrated by centrosomes, which are membrane-less organelles that nucleate and organize microtubules. Dysregulation of centrosome formation is lethal during embryogensis and can trigger tumor formation and metastasis in somatic cells. We want to understand how centrosomes assemble and nucleate microtubules through bottom-up reconstitution.