Interventional molecular systems, methods, and devices
to understand and improve human health
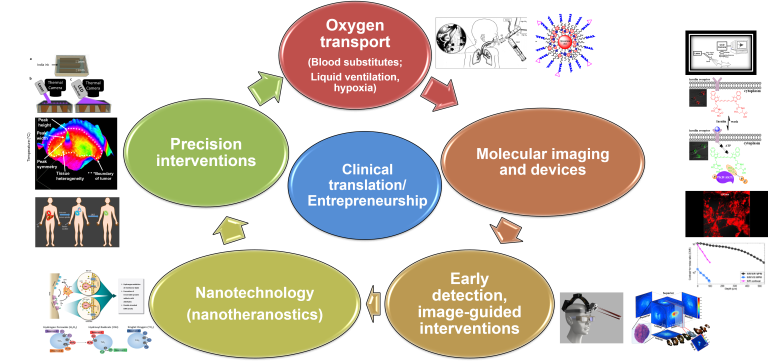
Mission Overview
There are major advances to be made in how we address diseases like cancer or neurological conditions. However, many diseased and healthy tissues are hard visualize within the body, presenting a major challenge for making these advances. We develop techniques using light and custom molecular contrast agents to visualize tissue inside animals and humans. We then use those tools to better understand the underlying biology on a molecular level, make diagnosis tools that are more accurate and less invasive than existing methods, and develop novel light-based treatment for disease.
Imaging is in the eye of the viewer
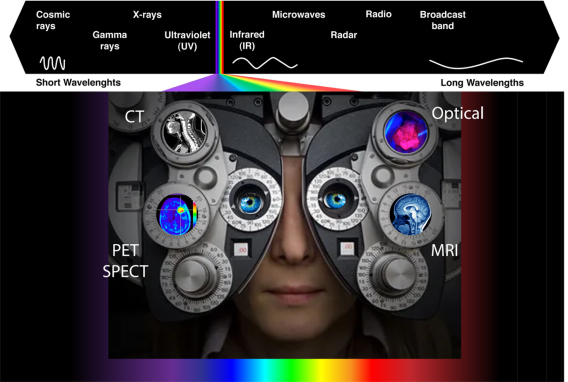
Optical Imaging in Medicine
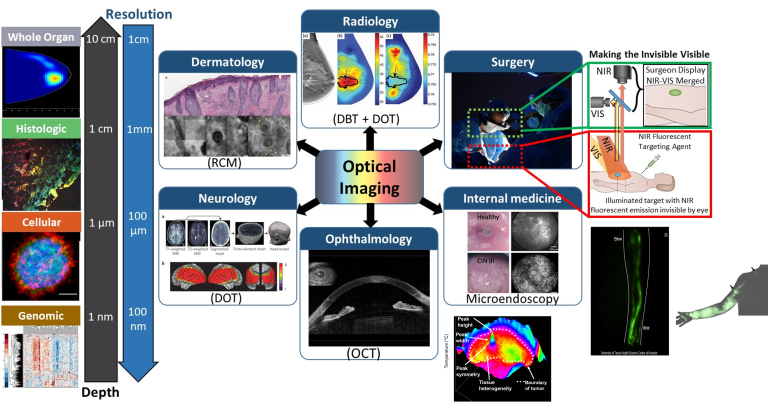
Application areas
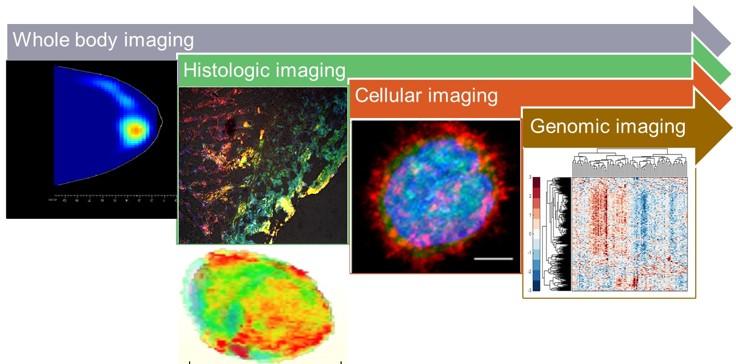
Innovative molecular imaging
The heterogeneity and continuous genetic adaptation of tumours complicate their detection and treatment via the targeting of genetic mutations. However, hallmarks of cancer such as aberrant protein phosphorylation and calcium-mediated cell signalling provide broadly conserved molecular targets. Here, we show that, for a range of solid tumours, a cyclic octapeptide labeled with a near-infrared dye selectively binds to phosphorylated Annexin A2 (pANXA2), with high affinity at high levels of calcium. Because of cancer-cell-induced pANXA2 expression in tumour-associated stromal cells, the octapeptide preferentially binds to the invasive edges of tumours and then traffics within macrophages to the tumour’s necrotic core. As proof-of-concept applications, we used the octapeptide to detect tumour xenografts and metastatic lesions, and to perform fluorescence-guided surgical tumour resection, in mice. Our findings suggest that high levels of pANXA2 in association with elevated calcium are present in the microenvironment of most solid cancers. The octapeptide might be broadly useful for selective tumour imaging and for delivering drugs to the edges and to the core of solid tumours.
Find more on the publication
Multidimensional reactive oxygen species generation
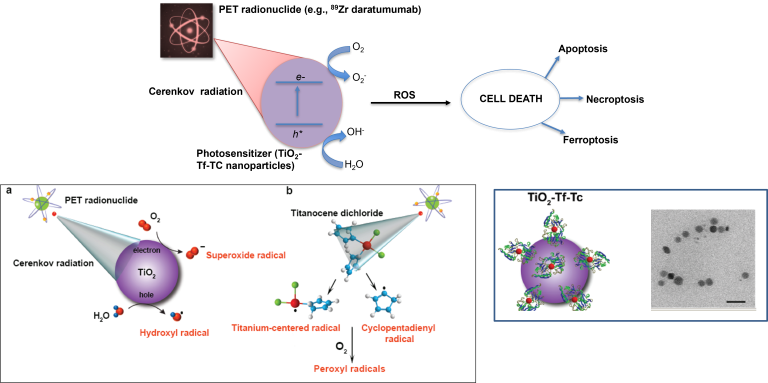
The combination of light and photosensitizers for phototherapeutic interventions, such as photodynamic therapy, has transformed medicine and biology. However, the shallow penetration of light into tissues and the reliance on tissue oxygenation to generate cytotoxic radicals have limited the method to superficial or endoscope-accessible lesions. Here we report a way to overcome these limitations by using Cerenkov radiation from radionuclides to activate an oxygen-independent nanophotosensitizer, titanium dioxide (TiO2). We show that the administration of transferrin-coated TiO2 nanoparticles and clinically used radionuclides in mice and colocalization in tumours results in either complete tumour remission or an increase in their median survival. Histological analysis of tumour sections showed the selective destruction of cancerous cells and high numbers of tumour-infiltrating lymphocytes, which suggests that both free radicals and the activation of the immune system mediated the destruction. Our results offer a way to harness low-radiance-sensitive nanophotosensitizers to achieve depth-independent Cerenkov-radiation-mediated therapy.
Find more on the publication
Cancer Vision Goggle
Evolution from static to dynamic label-free thermal imaging has improved bulk tissue characterization, but fails to capture subtle thermal properties in heterogeneous systems. Here, we report a label-free, high speed, and high-resolution platform technology, focal dynamic thermal imaging (FDTI), for delineating material patterns and tissue heterogeneity. Stimulation of focal regions of thermally responsive systems with a narrow beam, low power, and low cost 405 nm laser perturbs the thermal equilibrium. Capturing the dynamic response of 3D printed phantoms, ex vivo biological tissue, and in vivo mouse and rat models of cancer with a thermal camera reveals material heterogeneity and delineates diseased from healthy tissue. The intuitive and non-contact FDTI method allows for rapid interrogation of suspicious lesions and longitudinal changes in tissue heterogeneity with high-resolution and large field of view. Portable FDTI holds promise as a clinical tool for capturing subtle differences in heterogeneity between malignant, benign, and inflamed tissue.
Find more on the publication
Laser Stimulated Thermal Imaging
Evolution from static to dynamic label-free thermal imaging has improved bulk tissue characterization, but fails to capture subtle thermal properties in heterogeneous systems. Here, we report a label-free, high speed, and high-resolution platform technology, focal dynamic thermal imaging (FDTI), for delineating material patterns and tissue heterogeneity. Stimulation of focal regions of thermally responsive systems with a narrow beam, low power, and low cost 405 nm laser perturbs the thermal equilibrium. Capturing the dynamic response of 3D printed phantoms, ex vivo biological tissue, and in vivo mouse and rat models of cancer with a thermal camera reveals material heterogeneity and delineates diseased from healthy tissue. The intuitive and non-contact FDTI method allows for rapid interrogation of suspicious lesions and longitudinal changes in tissue heterogeneity with high-resolution and large field of view. Portable FDTI holds promise as a clinical tool for capturing subtle differences in heterogeneity between malignant, benign, and inflamed tissue.
Find more on the publication
Funding
-
National Institutes of Health (NIBIB and NCI)
-
National Science Foundation
-
Department of Defense Breast Cancer Research Program
-
CPRIT Established Investigator Award (RR220013)
Perspective
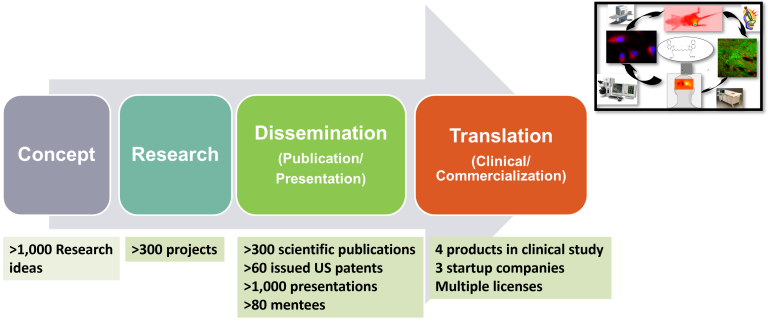
Concept
- >1,000 Research ideas
Research
- >300 projects
Dessemination
(Publication/Presentation)
- >300 scientific publications
- >60 issued US patents
- >1,000 presentations
- >80 mentees
Translation
(Clinical/Commercialization)
- 4 products in clinical study
- 3 startup companies
- Multiple licenses