We are interested in how metabolism can be controlled by intrinsic factors like the presence of substrate, product, or co-factors. The TCA cycle, a key pathway for energy production and biosynthesis, achieves exquisite control through these metabolic mechanisms.
In addition to the regulation of metabolism by gene expression, mechanisms exist that allow pathways to respond to acute metabolic conditions. Common metabolic mechanisms that contribute to metabolic regulation are substrate supply, redox state, and cofactor availability.
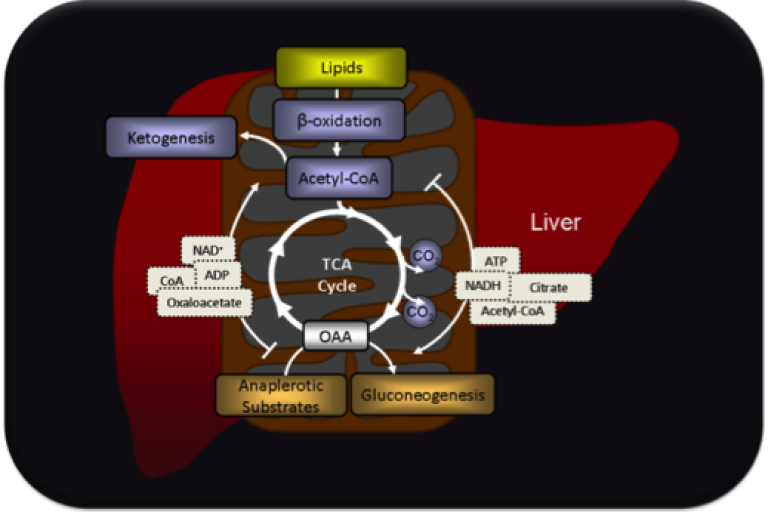
These mechanisms are especially important for complex pathways that traverse multiple metabolic systems or cellular compartments and depend on substrate supply by other tissue. Hepatic gluconeogenesis uses metabolic regulation to coordinate glucose synthesis with substrate supply from peripheral tissues and energy production in the hepatocyte.
Most gluconeogenic substrates must be transported into mitochondria, undergo anaplerosis into the tricarboxylic acid cycle, and then be transported out of the mitochondria before being converted to glucose. The process is energetically expensive and, thus, requires ancillary coordination with energy production through fat oxidation, TCA cycle metabolism, and respiration.
The state of these supporting pathways effect metabolic factors that influence gluconeogenic flux.
We are studying how pathways of substrate delivery, transport, anaplerosis, cataplerosis, and energy metabolism act in conjunction with molecular mechanisms to regulate gluconeogenesis and other biosynthetic pathways that are abnormal during disease.
References
McCommis KS, Chen Z, Fu X, McDonald WG, Colca JR, Kletzien RF, et al. Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling. Cell Metab. 2015. PMID: 26344101.
Gray LR, Sultana MR, Rauckhorst AJ, Oonthonpan L, Tompkins SC, Sharma A, et al. Hepatic Mitochondrial Pyruvate Carrier 1 Is Required for Efficient Regulation of Gluconeogenesis and Whole-Body Glucose Homeostasis. Cell Metab. 2015. PMID: 26344103.
Vigueira PA, McCommis KS, Schweitzer GG, Remedi MS, Chambers KT, Fu X, McDonald WG, Cole SL, Colca JR, Kletzien RF, Burgess SC, Finck BN. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 2014;7(6):2042-53. PMID: 24910426.
Mendez-Lucas A, Duarte JA, Sunny NE, Satapati S, He T, Fu X, Bermudez J, Burgess SC, Perales JC. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. J Hepatol. 2013;59(1):105-13. PMID: 23466304.
Wan M, Leavens KF, Hunter RW, Koren S, von Wilamowitz-Moellendorff A, Lu M, et al. A noncanonical, GSK3-independent pathway controls postprandial hepatic glycogen deposition. Cell Metab. 2013;18(1):99-105. PMID: 23823480; PMCID:3725134.
Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5(4):313-20. PMID: 17403375.