Understanding how mechanical force influences lung development
When babies take their first breath, a remarkable process occurs in which their lungs open and transition to the oxygen-rich environment we all live in. However, in babies that are born prematurely, their lungs are underdeveloped and may need help to function appropriately. In babies that are born at the limits of viability (22-24 weeks gestation), their lungs are severely underdeveloped and often require mechanical ventilation, which exposes the lung to increased biophysical force. In the Callaway lab, we aim to better understand how biophysical force impacts the developing lung and how this may predispose to the life-long lung disease bronchopulmonary dysplasia (BPD).
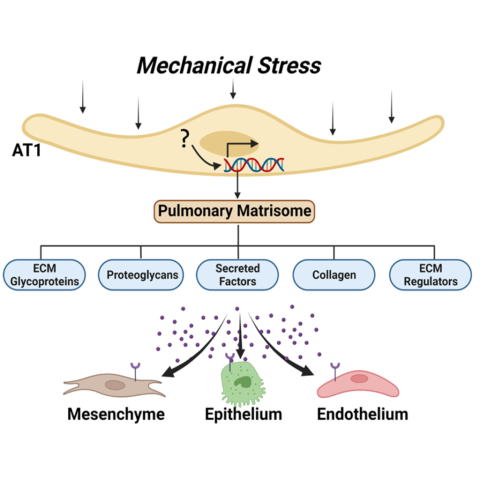
Type 1 pulmonary epithelial (AT1) cells encompass 95% of the alveolar surface area and are responsible for allowing gas exchange between the outside environment and our blood. Although this has been largely thought to be their primary function, more recently, the lung developmental biology community has identified their role as regulators of the pulmonary matrisome, or collection of extracellular matrix- (ECM) related constituents, proteins, and secreted factors. In fact, there are some ECM constituents that are unique to the AT1 cells and may serve as important hubs for communication with other cells of the developing lung. Our research goal is to identify how mechanical force impacts the AT1 cell transcriptional and translational program and overall lung development. To accomplish this, we utilize a neonatal mouse mechanical ventilation technique, unique transgenic mouse models, and cutting-edge imaging and informatics techniques. Understanding more about how the lung responds to acute mechanical force-related injury and remodels as a consequence of this injury leading to long-term structural changes may lead to a better understanding of BPD pathogenesis and highlight targets for intervention.