Objective
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. While treatment is effective when first diagnosed, limited options exist upon relapse. T-cells have been successfully engineered ex-vivo to express a Chimeric Antigen Receptor (CAR) to target and kill ALL cancer cells. Clinical trials in children with ALL relapse showed >80% efficacy.
The purpose of our effort is to develop a microbubble (MB)-based ultrasound gene delivery system to produce CAR-T cells in vivo, avoiding the use of viral vectors and the costly and time-consuming ex-vivo manipulation.
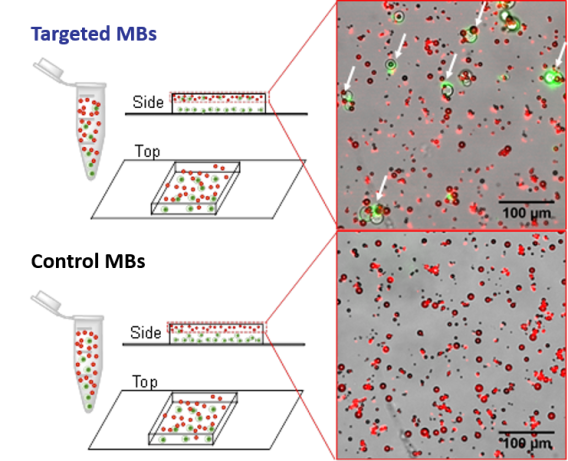 Following end-over-end mixing, samples are placed on a microscopy slide inside a thin silicone barrier to keep fluid in place and increase the volume of the well. Only targeted MBs show the association (arrows) with Jurkat cells (green).
Following end-over-end mixing, samples are placed on a microscopy slide inside a thin silicone barrier to keep fluid in place and increase the volume of the well. Only targeted MBs show the association (arrows) with Jurkat cells (green). Methods
MBs of perfluorobutane gas encapsulated with a PEGylated lipid shell, to which we added DSTAP, a positively charged lipid to attach DNA (GFP-plasmid), and longer chain PEG to attach either anti-CD3 antibody (a T cell marker) or a non-specific IgG as control. MBs were labeled with DiD, a red fluorescent dye and the final concentration, size, antibody and DNA loading quantified. T-cells (Jurkat cell) labeled with DiO, a green fluorescent dye, were used to assess and quantify binding efficiency.
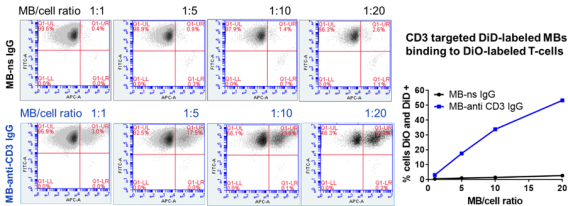 DNA loaded, anti-CD3 targeted MBs bound to > 50% of T-cells vs. <2% for control MBs.
DNA loaded, anti-CD3 targeted MBs bound to > 50% of T-cells vs. <2% for control MBs. Results
We show the successful targeting of T-cells with DNA loaded MBs. The next step is to optimize T-cell targeting in whole blood to assess targeting sensitivity and specificity and then in vitro transfection.