Acoustic droplet vaporization (ADV) of superheated perfluorocarbon nanodroplets (NDs) holds significant promise as an extravascular ultrasound contrast agent, with potential to advance ultrasound-guided therapeutic applications such as targeted drug delivery. Our research focuses on the design and formulation of NDs by exploring composition–structure–property relationships, aiming to expand diagnostic and therapeutic capabilities beyond the current limitations of microbubble-based technologies. We are actively pursuing several exciting projects in this area and welcome inquiries from those interested in joining our team or exploring collaborative opportunities.
Selected Publications
Direct emulsification of stable superheated perfluorobutane nanodroplets by sonication: addressing the limitations of the microbubble condensation technique, Ultrasound in Medicine and Biology, 2024, 50, 445-452. https://doi.org/10.1016/j.ultrasmedbio.2023.12.008
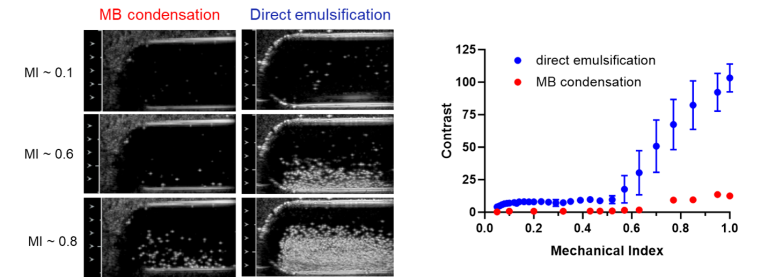
The dominant conclusion of this report is that nearly 100% of particles produced by direct emulsification of perfluorobutane (PFB) liquid at low temperature are PFB-filled, whereas the emulsion produced by microbubble (MB) condensation if the MBs are not washed, contains 2000 times more non-PFB filled than PFB-filled particles.
In Vivo Ultrasound Imaging of Macrophages Using Acoustic Vaporization of Internalized Superheated Nanodroplets, ACS Applied Materials and Interfaces, 2023, 15, 42413-42423. https://pubs.acs.org/doi/10.1021/acsami.3c11976

Positively charged nanodroplets containing low boiling point perfluorocarbon are internalized by macrophages and vaporize after being exposed to ultrasound pulses using a clinical scanner. Vaporization into microbubbles occurs without affecting cell survival or phagocytic function and is successfully achieved for at least 8 h after internalization both in vitro and in vivo.
Bubble Inflation Using Phase-Change Perfluorocarbon Nanodroplets as a Strategy for Enhanced Ultrasound Imaging and Therapy, Langmuir, 2020, 36, 2954-2965. https://pubs.acs.org/doi/10.1021/acs.langmuir.9b03647
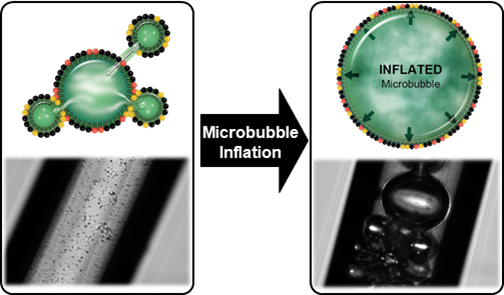

A new theory and method to inflate PFC gas bubbles have been developed and validated. This work demonstrates that the coexistence of liquid PFB NDs and gaseous PFB MBs leads to the dramatic inflation of the gas bodies, increasing their diameter by at least 2 orders of magnitude.
Fluorous-Phase Iron Oxide Nanoparticles as Enhancers of Acoustic Droplet Vaporization of Perfluorocarbons with Supra-Physiologic Boiling Point, J Control Release, 2019, 302, 54-62. https://www.sciencedirect.com/science/article/pii/S0168365919301592
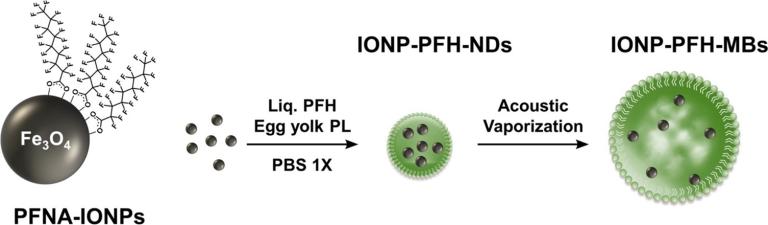
Using Perfluorohexane nanodroplets (PFH-NDs) loaded with 5% w/v iron oxide nanoparticles (IONPs), we were able to trigger acoustic droplet vaporization (ADV) at physiological temperature and at 42 °C using pressures that are just above the FDA-approved limit for clinical ultrasound scanners. ADV threshold for the 10 and 15% IONP emulsions at 42 °C were lower than the FDA limit. When IONP-PFH-NDs containing 5% IONP that passively accumulated in tumors by 5 h after IV administration were exposed to an ADV pulse in vivo, they converted to MBs detectable by ultrasound. Our findings are in agreement with the known concept that particles within a liquid provides nucleation sites that lower the ultrasound cavitation threshold
Novel Method for Formation of Monodisperse Superheated Perfluorocarbon Nanodroplets as Activatable Ultrasound Contrast Agents, RSC Advances, 2017, 7, 48561-48568. https://pubs.rsc.org/en/content/articlelanding/2017/ra/c7ra08971f

This work confirms that direct emulsification of low boiling point perfluorocarbon (such as perfluorobutane or “decafluorobutane” (DFB) and octafluoropropane (OFP)) into liquid nanodroplets for phase-shift ultrasound controlled vaporization is possible. Emulsions of DFB were stable for >18 days (entire observation periods) at 4 °C and >1 day at room temperature allowing further processing for functionalization and purification. More important, DFB formulations were stable for at least 2 h at physiologic temperature without spontaneous vaporization, allowing ample time for targeting and tissue accumulation. They transitioned into MBs in vitro only when exposed to ultrasound at low Peak Negative Pressure (PNP = 0.38 for OFP and 1.07 for DFB) producing marked enhancement on B-mode US imaging.