Overview
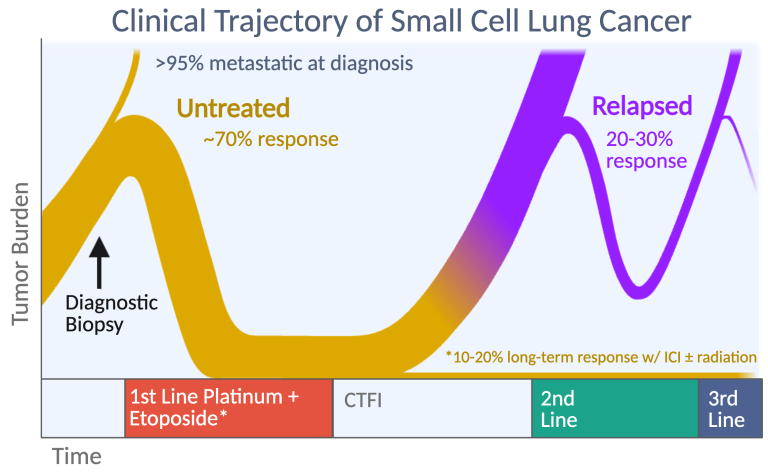
Small cell lung cancer (SCLC) is a common and aggressive disease with a dismal prognosis that has not improved significantly in the past 40 years. Since the early 1970’s, thoracic oncologists have separated SCLC from other lung tumors, collectively termed “non-small cell lung cancer” (NSCLC), because of striking differences in its initial presentation and subsequent response to therapy. Our research focuses on three major problems that distinguish SCLC from NSCLC: (1) extreme sensitivity to fist-line chemotherapy followed by acquired chemoresistance, (2) early metastasis that renders more than 95% of cases inoperable, and (3) the absence of oncogenic mutations that can be inhibited with targeted therapies. These clinical obstacles render SCLC recalcitrant to therapy and rapidly fatal.
Drivers of Chemoresistance in SCLC
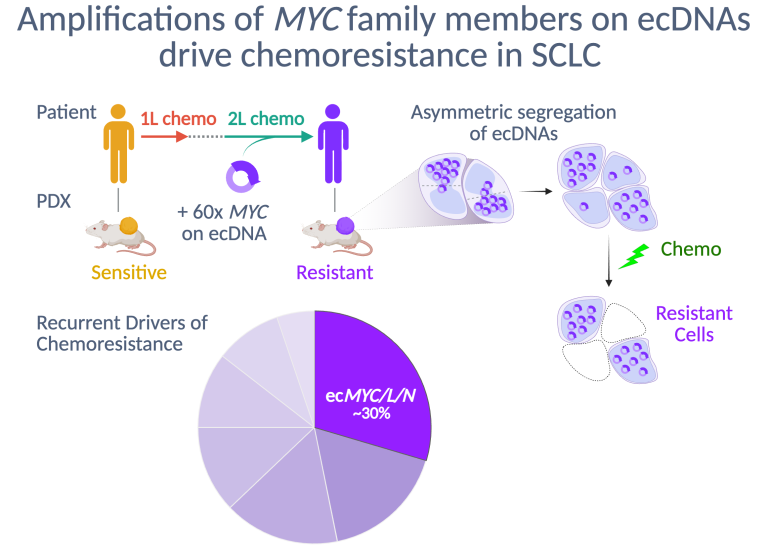
Platinum-based chemotherapy is recommended for all SCLC patients, regardless of stage, and almost always causes dramatic tumor regressions. However, these regressions are transient, and after relapse SCLC usually acquires broad resistance to chemotherapy. We found that patient-derived xenograft (PDX) models of SCLC faithfully recapitulate this transformation from chemosensitive to chemoresistant disease. We discovered that high-level amplifications of MYC or its paralogs, MYCN or MYCL, on circular extrachromosomal DNA (ecDNA) are recurrent in chemoresistant PDX models from patients with relapsed SCLC. Within ecMYC+ SCLC tumors, ecDNAs segregate randomly at mitosis, and the cells that inherit the highest copy numbers are most resistant. Our current research is focused on discovering the mechanism by which ecMYC drives chemoresistance, new strategies to overcome this resistance, and the drivers of chemoresistance in SCLC that lacks ecMYC/L/N amplifications.
Brain Metastasis in SCLC
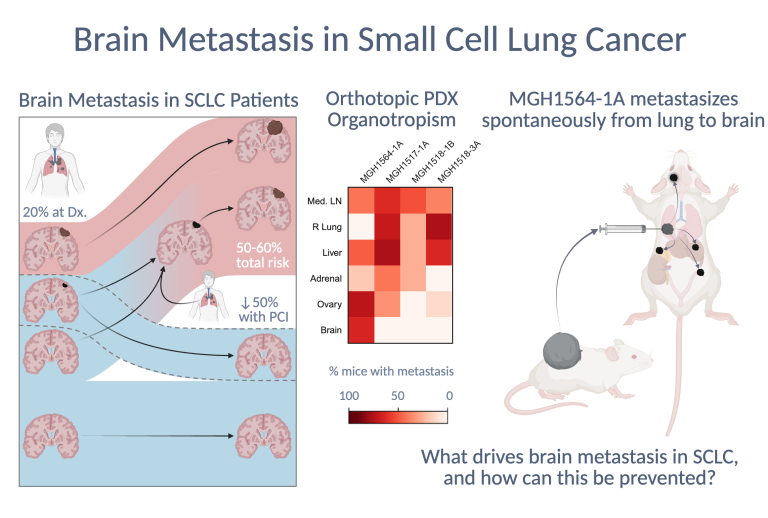
SCLC is almost always metastatic at diagnosis, and in most cases spreads to the brain, often causing severe neurologic morbidity in addition to mortality. Importantly, most brain metastases (BMs) emerge after diagnosis, meaning that there is an opportunity for interception or prevention. However, there are no reported models of SCLC that metastasize spontaneously to the brain. We recently optimized a surgical protocol for orthotopic transplantation of dissociated patient-derived xenograft (PDX) cells into the left lungs of mice. Two of these orthotopic models generated spontaneous and highly recurrent BMs that caused model-specific neurologic complications. Our current research focuses on discovering the molecular features and drivers of brain organotropism in SCLC to prevent or treat brain metastasis.
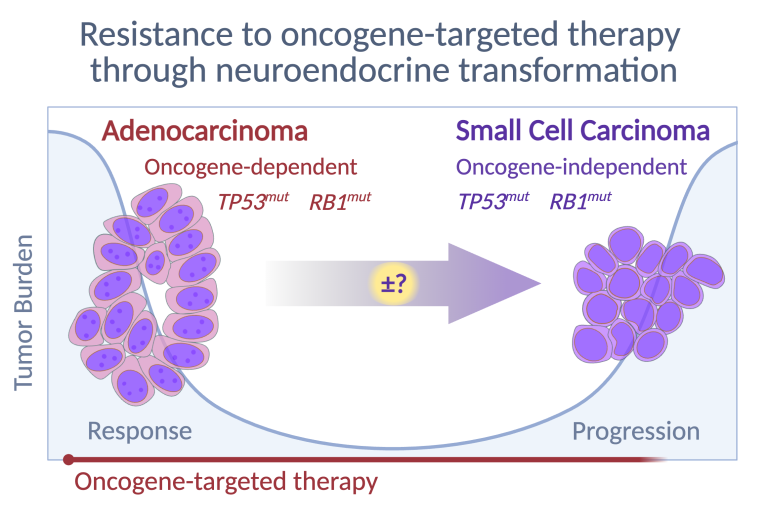
Neuroendocrine Transformation of Adenocarcinoma
Loss of RB1 and TP53 are hallmarks of SCLC, but the oncogenic drivers remain unclear, as the absence of premalignant lesions or defined cells of origin complicates the development of targeted therapies. Non-small cell lung cancers (NSCLCs) differ significantly from SCLC in embryology, histology, mutations, and clinical management, yet exhibit surprising plasticity – evident when EGFR-mutant NSCLC transforms into treatment-resistant SCLC (t-SCLC) upon loss of RB1 and TP53. Unlike de novo SCLC, the cell of origin for t-SCLC is known, facilitating research into transformation. We focus particularly on ASCL1, a neurodevelopmental transcription factor identified by Jane Johnson at UT Southwestern, as evidence strongly suggests it drives NSCLC-to-SCLC conversion. ASCL1, rarely expressed in other malignancies, is essential for SCLC viability, drives tumorigenesis in mouse models, and becomes activated following transdifferentiation in EGFR-mutant NSCLC. Our research seeks to define factors triggering ASCL1 activation in lung adenocarcinoma cells and mechanisms enabling ASCL1-driven neuroendocrine transformation and EGFR-independence.