Autophagy is a universally conserved process employed by eukaryotic cells to degrade and reutilize the constituents of cytoplasm and organelles. Both extremely low and high autophagy activity is associated with human disease. My research laboratory closely collaborates with Dr. Beth Levine to examine the effects of autophagy on acute kidney injury, chronic kidney disease, uremic cardiomyopathy, and aging.
We are the first to demonstrate that Klotho is able to upregulate autophagic flux—likely through disruption of beclin 1/BCL2 complex—one of the mechanisms whereby Klotho exerts its renoprotection in acute kidney injury model. We also confirmed an increase of autophagic flux in multiple organs of knock-in gain-of-function beclin 1 mice. This increased autophagy activity effectively rescues aging and aging-associated phenotypes in our premature aging mouse, hypomorphic Klotho allele (kl/kl), that Dr. Makoto Kuro-o generated.
Now, we are working on whether (and how) the interplay between Klotho and autophagy improves phosphate homeostasis, and how it reduces hyperphosphatemia and high phosphate associated diseases—phosphotoxicity— in Chronic Kidney Disease.
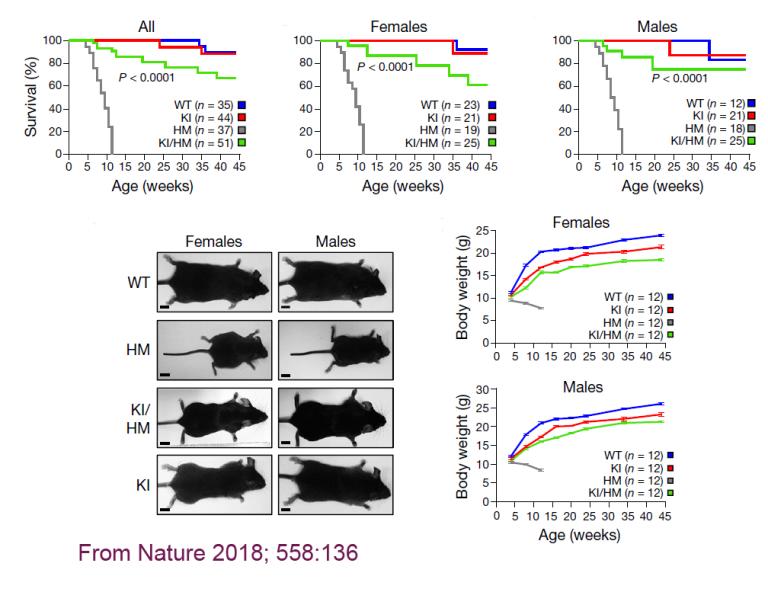 Expression of beclin 1 F121A prevents lethality of Klotho-deficient mice
Expression of beclin 1 F121A prevents lethality of Klotho-deficient mice