Alzheimer's disease is the most common cause of dementia in the elderly and remains a devastating disease with currently no cure and limited therapeutic options.
The exact pathogenesis of Alzheimer’s disease is being actively investigated, but a leading hypothesis is that the abnormal accumulation of amyloid-beta peptides is a major contributor to the development of dementia. While deficits in cognition and memory are the major clinical manifestations of Alzheimer’s disease, accelerated early body weight loss often occurs prior to mental decline. Furthermore, weight loss is correlated with worsening disease progression and increased risk of death in Alzheimer’s disease. Therefore, brain circuits regulating body weight may be altered early in Alzheimer’s disease and could be intrinsic to the disease process. Our laboratory is interested in identifying the central and peripheral pathways regulating body weight and systemic metabolism that are altered early in Alzheimer’s disease with a focus on the hypothalamus. Additionally, we are also interested in examining how hypothalamic dysfunction in AD could result in cognitive and other non-cognitive manifestations of AD. We explore these questions using a wide range of techniques from molecular biology, genetics, genomics, electrophysiology, and neuroimaging approaches.
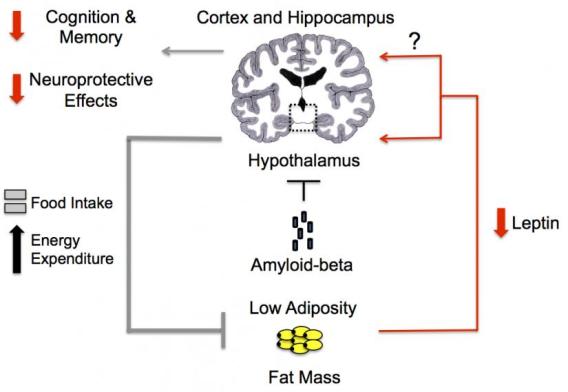
Schematic of a proposed pathophysiological mechanism for amyloid-beta mediated inhibition of hypothalamic pathways leading to weight loss and a low leptin state.
In Alzheimer’s disease, non-cognitive manifestations including body weight loss often occur early before any significant cognitive decline. We hypothesize that amyloid-beta and other pathogenic factors of Alzheimer’s disease can disrupt brain circuits that regulate these non-cognitive manifestations early in the disease process. Supporting this hypothesis, in mouse models, we have identified key hypothalamic neurons important for body weight homeostasis that are susceptible to the pathological effects of amyloid-beta prior to the development of memory impairment. Interestingly, not all hypothalamic neurons were found to be adversely affected by amyloid-beta. This suggests that there are selectively vulnerability cell populations in the hypothalamus to Alzheimer’s disease pathology. Ongoing studies seek to identify the hypothalamic neuronal populations and the pathways affected early by Alzheimer’s disease pathology and how they may contribute to the development of non-cognitive manifestations of Alzheimer’s disease.
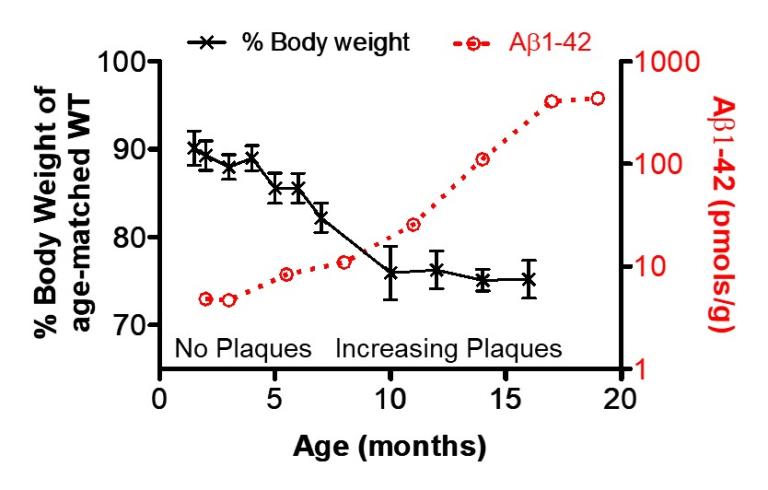
Increasing brain amyloid-beta1-42 burden correlates with worsening body weight deficits in Tg2576mice (Adapted from Ishii, M. et al., J Neuroscience 2014)
Understanding how amyloid-beta causes dysfunction in hypothalamic neurons is an active area of investigation. We have identified a novel mechanism for amyloid-beta mediated dysfunction of hypothalamic neurons through L-type calcium channels, where amyloid-beta caused a shift from high to low voltage-threshold activated L-type calcium channel. Ongoing studies continue to investigate the molecular and cellular mechanisms underlying amyloid-beta mediated hypothalamic dysfunction with the goal of developing potential therapeutic interventions based on these findings.
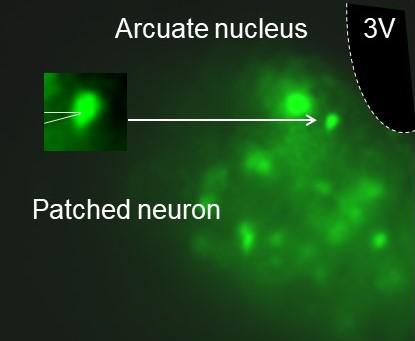
GFP labeled neuropeptide Y (NPY) neurons in the arcuate nucleus of the hypothalamus are evaluated by whole-cell patch clamp.
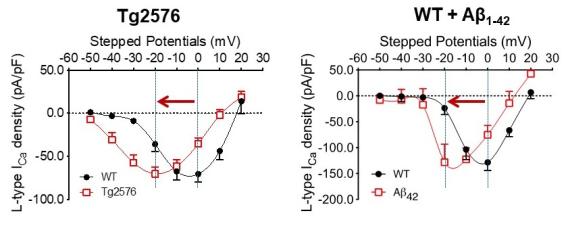
L-type calcium currents in the arcuate NPY neurons from Tg2576 or wildtype slices treated with exogenous amyloid-beta1-42 show a propensity for channel opening close to the resting potential (Adapted from Ishii, M. et al., J. Neuroscience 2019).
A major focus of the laboratory is to verify the findings from the mechanistic studies and to identify additional cardiometabolic signaling factors altered early in Alzheimer’s disease in clinically relevant human studies. We have previously found that body mass index (BMI) is negatively associated with Alzheimer’s disease pathology in cognitively normal older adults. Furthermore, select circulating levels of adipokines (adipocyte-derived hormones) are altered during the preclinical stage of Alzheimer’s disease, where cognition is intact but there is evidence of Alzheimer’s disease pathology. Interestingly, these metabolic changes were found in men but not women, suggesting important sex differences early in the development of Alzheimer’s disease. Ongoing studies continue to evaluate potential biofluid and imaging markers to determine whether early changes in systemic metabolism and hypothalamic structure and function are evident in Alzheimer’s disease and their relation to amyloid-beta and tau pathology.
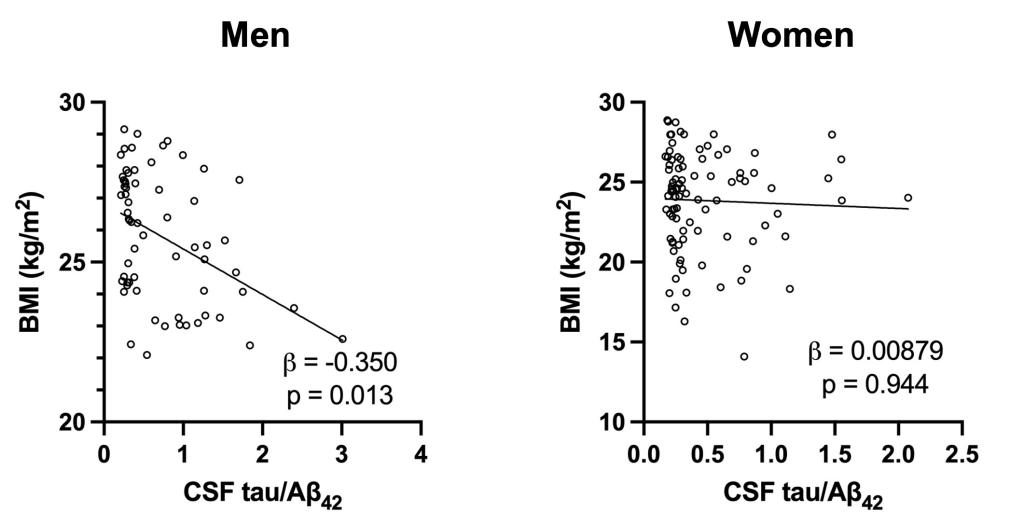
Body mass index (BMI) is significantly associated with cognitively intact older adult men but not women with Alzheimer’s disease pathology (Adapted from Eruysal, E., et al., Journal of Alzheimer’s Disease 2023).
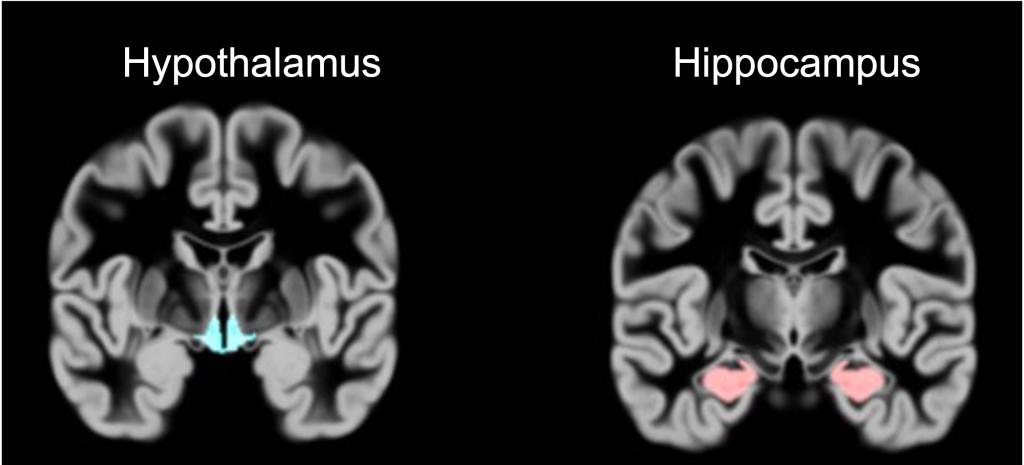
The hypothalamus in a human brain MRI scan