The Jain Lab is broadly interested in the impact of sex hormones, primarily estrogen on respiratory diseases and host immune responses to infections by agents such as Pseudomonas aeruginosa.
We focus much of our work on cystic fibrosis (CF) and non-CF bronchiectasis. CF is an autosomal recessive inherited disease. Morbidity and mortality are primarily driven by progressive airway obstruction, inflammation, and recurrent respiratory infections.
CF is equally prevalent in both sexes, but females with CF have a shortened life expectancy relative to males. In addition, female patients acquire respiratory infections at an earlier age than male and have a more rapid decline in health.
Similar patterns have been described in women versus men with other airway diseases such as chronic obstructive pulmonary disease, asthma, and non-CF-related bronchiectasis.
We particularly focus on how estrogen affects the interaction of the airway epithelium and neutrophils, as well as its effects on functional responses of neutrophils.
Our long-term goal is to build the foundation for novel hormone-modulating therapeutics or other treatments to narrow the sex disparity in these respiratory diseases.
We study the impact of sex hormones on neutrophil responses to Pseudomonas aeruginosa and the interaction of the epithelium and neutrophils under these conditions.
We have a particular interest in studying the effects of estrogen on neutrophil NETosis and degranulation.
We use murine models of acute and chronic infection with Pseudomonas aeruginosa in wild-type and CFTR-null mice to determine the impact of sex hormones on inflammation and bacterial clearance.
Male and female mice, both intact, ovariectomized or orchiectomized, undergo hormone manipulation to determine specific receptor influences and target pathways. We employ a array of techniques such as immunohistochemistry, flow cytometry, and RT-PCR.
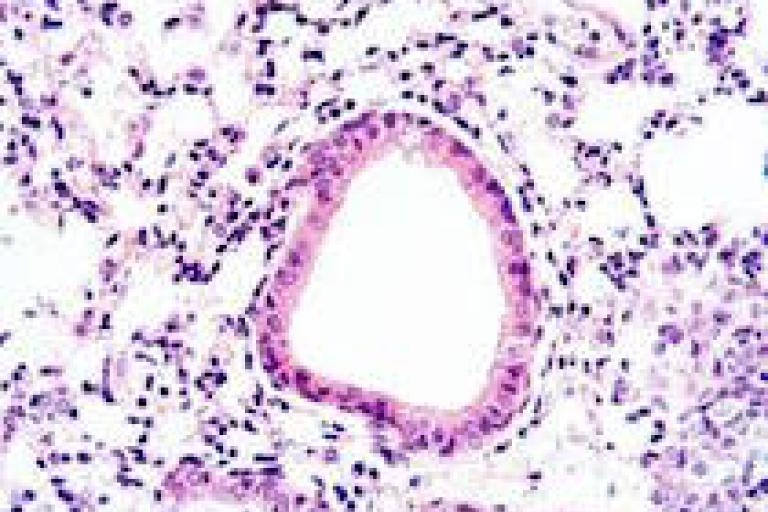
Image: Histology of lungs from our mouse model of Pseudomonas aeruginosa infection.
We participate in a number of multicenter clinical trials studying novel therapies for the treatment of cystic fibrosis and other forms of bronchiectasis. These therapies include anti-inflammatories, anti-infectives, and CFTR modulators in the form of Phase 1, 2, and 3 trials, both outpatient and inpatient.
We are also spearheading prospective clinical trials to understand the impact of natural hormone fluctuation, birth control, and pregnancy on markers of health in Cystic Fibrosis patients that correlate with our bench related studies.
See listings of these trials in:
- clinicaltrials.gov
- UTSW research trial database
- Cystic Fibrosis Foundation Drug Development Pipeline
Our adult Cystic Fibrosis Program is accredited by the Cystic Fibrosis Foundation. Primary ciliary dyskinesia foundation (hyperlink) and NTM and Bronchiectasis Association.
We participate in national registries and clinical trials in collaboration with these organizations.
Please contact our clinic at 214-645-0599 for further information, or visit our clinic website.
Our adult Cystic Fibrosis Program is accredited by the Cystic Fibrosis Foundation; our adult Primary Ciliary Dyskinesia Program is accredited by the Primary Ciliary Dyskinesia Foundation; our bronchiectasis program is also accredited by the NTM and Bronchiectasis Association. We serve over 700 adults with CF and bronchiectasis and have a multi-disciplinary team of physicians, nurses, respiratory therapists, social workers and dieticians dedicated to their care.
Please contact the clinic at (214) 645-0599 for more information.