Modulation of CEST by T1 relaxation
Chemical exchange saturation transfer (CEST) agents alter MR image contrast by transferring selectively saturated 1H spins from a small proton pool into the bulk water pool.
The lifetime of the bound water molecule in Eu3+-based PARACEST agents has been found to be quite sensitive to the electronic effects of the ligand pendant arms and alterations in the coordination geometry. The CEST effect in these systems also depends on the longitudinal relaxation time (T1) of the bulk water pool and this can be used to modulate the CEST signal.
We recently reported a Eu3+ (DOTA-tetraamide) complex containing two nitroxide free-radical groups. The nitroxide groups shortened the T1 of the bulk water protons which, in turn, quenched CEST signal originating from the Eu3+-bound water. Reduction of paramagnetic nitroxide moieties by L-ascorbate to a diamagnetic species “turns on” CEST.
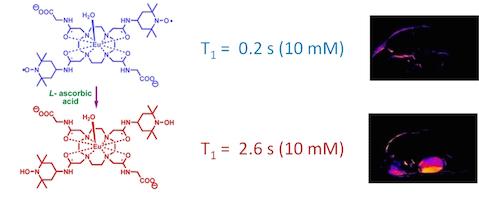 The CEST signal “turns on” on after in vivo reduction of the nitroxide radicals.
The CEST signal “turns on” on after in vivo reduction of the nitroxide radicals. Eu2+/3+DOTA(gly)4: a bridge between T1 shortening and paraCEST agents
T1 shortening agents such as paramagnetic metal salts (Gd3+ and Mn2+ complexes) influence image contrast by shortening the longitudinal (T1) and transversal (T2) relaxation times of water protons. Eu2+ is isoelectronic with Gd3+ (both have seven unpaired electrons in an 8S7/2 ground state) but Eu2+ complexes have faster water exchange kinetics than the corresponding Gd3+ complexes.
Most Eu2+ polyamino polycarboxylate complexes have a redox potential significantly lower than that of the Eu2+ aqua ion; Eu2+ complexes have been proposed as redox-sensitive T1 shortening agents because oxidation of Eu2+ yields weakly paramagnetic Eu3+.
Recently we have reported the Eu2+ complex of DOTA(gly)4 has different contrast-enhancing properties depending on the oxidation state of the metal. In its divalent form it is an efficient T1 shortening agent, but oxidation converts it into Eu3+DOTA(gly)4, which is a commonly used paraCEST agent.
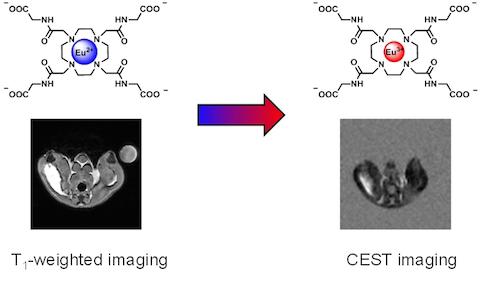 The T1 shortening effect diminishes while the CEST effect increases as Eu2+ is being oxidized to Eu3+ in vivo.
The T1 shortening effect diminishes while the CEST effect increases as Eu2+ is being oxidized to Eu3+ in vivo.