
Mechanisms of the Seasonal Clock
Seasonal rhythms such as hibernation, migration, and seasonal reproduction are widespread in nature. In humans, many aspects of normal physiology and disease, including seasonal affective disorder, cardiovascular diseases, and viral infections follow notable seasonal patterns (Fig. 1).
Classical work since the 1950s (Fig. 2) has revealed that seasonal rhythms, much like circadian rhythms, are driven by an endogenous clock. This “seasonal clock” (also termed circannual clock) is entrained by environmental cues like photoperiod, but continues to oscillate even in nonseasonal conditions, allowing organisms to anticipate and prepare for seasonal environmental changes.
Despite its fundamental importance, little is known about the molecular basis of the seasonal clock, largely due to the lack of a suitable genetic model. To address the gap, we have established a new seasonal primate model, the mouse lemur. Mouse lemurs exhibit striking annual cycles of body weight, torpor, and gonadal development, and maintain robust seasonal rhythms in lab colonies (Fig 3). Building on the resources and tools we previously developed for this model, we are currently combining single-cell omics profiling of the brain with forward phenotypic screening to search for the genes and regulatory circuits that underlie the seasonal clock.
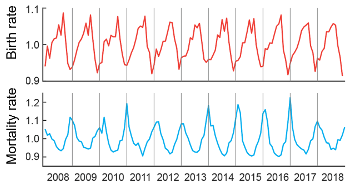
Fig 1. Human births and deaths follow persistent seasonal patterns.
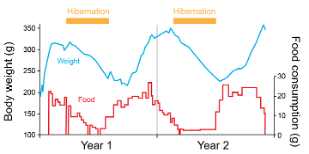
Fig 2. Ground squirrels continue free-running seasonal cycles of hibernation and body weight under non seasonal conditions. Adapted from: Pengelley & Fisher 1957 Nature, 1963 Can. J. Zool.
Seasonal Metabolism & Tissue Plasticity
Across the year, seasonal rhythms drive profound yet reversible physiological remodeling, from tissue architecture to metabolism and function. The mouse lemur, for example, undergoes significant annual cycles of body weight, fat storage, and seasonal reproductive organ growth and regression (Fig. 3). Many seasonal songbirds show striking annual cycles of adult neurogenesis and neuronal loss in the song-control circuits. Understanding how these extreme yet nonpathological changes occur provides a unique opportunity to uncover new mechanisms of metabolic control and tissue regeneration.
Although evident in select tissues, the scope of seasonal tissue remodeling remains unclear. Our work integrates cellular and system-level approaches to study these transformations. We use tissue imaging to resolve where seasonal tissue remodeling occurs and single-cell omics to uncover how the process is controlled within tissues by different cell types and regulatory networks. We also collaborate to profile metabolites and proteins in the circulation and key metabolic organs across seasons to identify the molecules driving seasonal metabolic reprogramming.
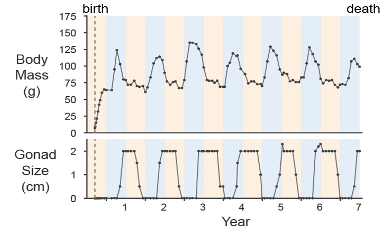
Fig 3. Seasonal cycles of body weight and gonad size in laboratory mouse lemurs.

Primate Model for Human Health
Model organisms have revolutionized biology, yet many crucial aspects of primate biology and disease are poorly represented in existing models. This limitation is well recognized in complex systems such as the brain and the immune system. The mouse lemur is the smallest and fastest-reproducing primates, and provides an attractive platform for studying primate genetics, evolution, and diseases that are difficult to approach in traditional models.
Our work on the Tabula Microcebus cell atlas and comparative analyses across lemur, human, and mouse identified molecular signatures of primate specialization and uncovered many genes for which lemur provides a closer human model than mouse (Fig. 4). Many of these genes are implicated in immune regulation and cancer. We are using computational and experimental approaches to explore this gene set, prioritizing key candidates for mechanistic studies of their roles in tumor development, immune evasion, and therapeutic translation.
We also collaborate to advance the mouse lemur as a model for sporadic Alzheimer’s disease (AD), addressing key limitations of rodent models. Like humans, aged mouse lemurs can spontaneously develop AD-like neurodegeneration, showing hallmarks of AD, including brain atrophy and widespread amyloid and tau pathologies. By comparing young, aged, and AD-like lemur brains with those of human and rodent models and by developing genetic and viral tools for targeted perturbation, we aim to identify primate mechanisms of neurodegeneration and resilience that are absent in rodents.
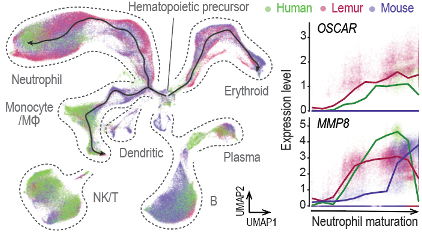
Fig 4. Evolutionary comparison of hematopoiesis identify genes with primate expression patterns. Left: Species-integrated UMAPs. Right: example genes with primate-selective expression patterns during neutrophil development.