Our focus is to identify mechanism(s) of autoimmunity in patient populations with diseases of the central nervous system (CNS) that will facilitate the development of therapies to prevent long-term deficits. Indeed, our work has already directly contributed to:
- Development of novel therapies in the treatment of multiple sclerosis (MS)
- Mechanistic understanding of existing therapies using animal models of neuroinflammation (Experimental Autoimmune Encephalitis, EAE).
- Initiation of clinical trials based on our finding that the cerebrospinal fluid of adults with MS contain highly activated B cells relevant to CNS inflammation and how B cell depletion reduces disease using the EAE model.
We were also the first to:
- Map the mechanism of action for glatiramer acetate on B cell activity in MS patients
- Identify the signaling modality that is dysregulated in the B cell pool of MS patients
- Discover and validate the first-ever biomarker of MS disease based on antibody genetics.
- Demonstrate antibodies produced by B cells from MS patients bind neurons in the cortex, supporting the concept of underlying gray matter disease as a critical feature of MS pathology in addition to white matter disease.
These seminal studies by our laboratory establish our experience in understanding the role of B cells and their antibody products in the mechanism(s) of brain inflammation in MS. We now use this experience to pursue our understanding of other diseases involving the adaptive immune system. As a result of our ground-breaking experience, my laboratory is well-equipped to conduct immunological and immunogenetics studies that lead the development of therapies that prevent long-term deficits in humans.
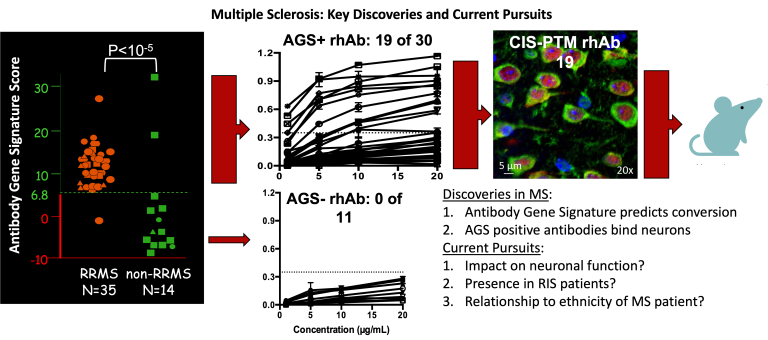
We were the first to discover that B cells in the Cerebrospinal fluid of MS patients produce antibodies that bind neurons. We successfully used single cell PCR technology established in my laboratory to generate 115 recombinant human antibodies from single B cells isolated from the cerebrospinal fluid of 42 adult patients experiencing their first clinical episode with neurological deficit that are later diagnosed with MS produce antibodies that bind neurons and that this binding only occurs when particular antibody genes are used by the B cell. We also demonstrated that CSF-derived B cells undergo extensive clonal expansion and somatic hypermutation accumulation. More recently, we have demonstrated that peripheral VH4+ plasmablasts from adult and pediatric MS patients also produce anti-neuronal antibodies.
Cameron, E. M., Spencer, S., Lazarini, J., Harp, C. T., Ward, E. S., Burgoon, M., Owens, G. P., Racke, M. K., Bennett, J. L., Frohman, E. M., & Monson, N. L. (2009). Potential of a unique antibody gene signature to predict conversion to clinically definite multiple sclerosis. Journal of Neuroimmunology, 213(1-2), 123–130.
Ligocki, A. J., Rounds, W. H., Cameron, E. M., Harp, C. T., Frohman, E. M., Courtney, A. M., Vernino, S., Cowell, L. G., Greenberg, B., & Monson, N. L. (2013). Expansion of CD27high plasmablasts in transverse myelitis patients that utilize VH4 and JH6 genes and undergo extensive somatic hypermutation. Genes and Immunity, 14(5), 291–301.
Lambracht-Washington, D., O'Connor, K. C., Cameron, E. M., Jowdry, A., Ward, E. S., Frohman, E., Racke, M. K., & Monson, N. L. (2007). Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid B cells from patients with relapsing remitting MS. Journal of Neuroimmunology, 186(1-2), 164–176.
Rivas, J. R., Ireland, S. J., Chkheidze, R., Rounds, W. H., Lim, J., Johnson, J., Ramirez, D. M., Ligocki, A. J., Chen, D., Guzman, A. A., Woodhall, M., Wilson, P. C., Meffre, E., White, C., 3rd, Greenberg, B. M., Waters, P., Cowell, L. G., Stowe, A. M., & Monson, N. L. (2017). Peripheral VH4+ plasmablasts demonstrate autoreactive B cell expansion toward brain antigens in early multiple sclerosis patients. Acta Neuropathologica, 133(1), 43–60.
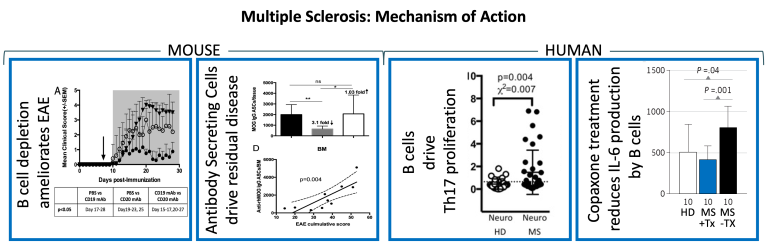
We were the first team to suggest that B cell depletion therapy (BCDT) might be efficacious in the treatment of Multiple Sclerosis and to demonstrate that Rituximab, a B cell depleting antibody could impact B cell frequencies in the cerebrospinal fluid and periphery of patients with the primary progressive form of MS. We also used mouse models of experimental autoimmune encephalomyelitis (EAE) to demonstrate that a subset of B cells called plasmablasts drive residual disease in animals treated with Rituximab, an effect that can be reversed by antibodies that selectively deplete these plasmablasts. We have also demonstrated that peripheral B cells from MS patients drive Th17 development and proliferation in response to neuro-antigens, whereas B cells from healthy donors do not. We also demonstrated that Copaxone therapy can reverse the CD40-mediated hyper-proliferation by B cells from MS patients and significantly reduce the robust amounts of IL-6 secreted by treatment-naïve B cells from MS patients.
Ireland, S. J., Guzman, A. A., Frohman, E. M., & Monson, N. L. (2016). B cells from relapsing remitting multiple sclerosis patients support neuro-antigen-specific Th17 responses. Journal of Neuroimmunology, 291, 46–53.
Chen, D., Ireland, S. J., Davis, L. S., Kong, X., Stowe, A. M., Wang, Y., White, W. I., Herbst, R., & Monson, N. L. (2016). Autoreactive CD19+CD20- Plasma Cells Contribute to Disease Severity of Experimental Autoimmune Encephalomyelitis. Journal of Immunology (Baltimore, Md. : 1950), 196(4), 1541–1549.
Chen, D., Ireland, S. J., Remington, G., Alvarez, E., Racke, M. K., Greenberg, B., Frohman, E. M., & Monson, N. L. (2016). CD40-Mediated NF-κB Activation in B Cells Is Increased in Multiple Sclerosis and Modulated by Therapeutics. Journal of Immunology (Baltimore, Md. : 1950), 197(11), 4257–4265.
Ireland, S. J., Guzman, A. A., O'Brien, D. E., Hughes, S., Greenberg, B., Flores, A., Graves, D., Remington, G., Frohman, E. M., Davis, L. S., & Monson, N. L. (2014). The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA Neurology, 71(11), 1421–1428.
Harp, C. T., Lovett-Racke, A. E., Racke, M. K., Frohman, E. M., & Monson, N. L. (2008). Impact of myelin-specific antigen presenting B cells on T cell activation in multiple sclerosis. Clinical Immunology (Orlando, Fla.), 128(3), 382–391.
Monson, N. L., Cravens, P. D., Frohman, E. M., Hawker, K., & Racke, M. K. (2005). Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Archives of Neurology, 62(2), 258–264.
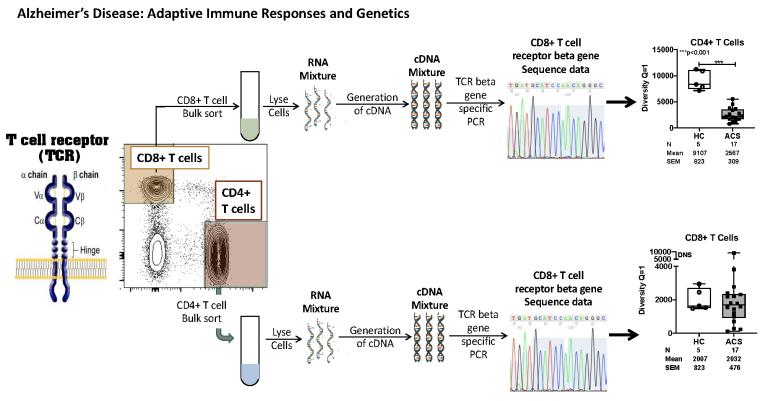
We were the first to discover that innate cells were expanded in the CSF of AD patients. We put together a team with expertise in immunology, neuroscience and vascular physiology to address this further. This team has published 4 papers with non-federal funding to demonstrate that innate cells are expanded in the CSF of patients with preclinical AD, that increases of Abeta deposition in the brain correlates with a decrease in B cells, and that physical activity can rescue some of this impact on brain health. We have more recently shown that CD4+ T cells undergo extensive clonal expansion in the CSF of AD patients and use particular T cell receptor genes that can distinguish patients with dementia from similar-aged healthy donors.
Monson, N. L., Ireland, S. J., Ligocki, A. J., Chen, D., Rounds, W. H., Li, M., Huebinger, R. M., Munro Cullum, C., Greenberg, B. M., Stowe, A. M., & Zhang, R. (2014). Elevated CNS inflammation in patients with preclinical Alzheimer's disease. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 34(1), 30–33.
Joshi, C., Sivaprakasam, K., Christley, S., Ireland, S., Rivas, J., Zhang, W., Sader, D., Logan, R., Lambracht-Washington, D., Rosenberg, R., Cullum, M., Hitt, B., Li, Q. Z., Barber, R., Greenberg, B., Cowell, L., Zhang, R., Stowe, A., Huebinger, R., Kelley, B., … Monson, N. (2021). CSF-Derived CD4+ T-Cell Diversity Is Reduced in Patients With Alzheimer Clinical Syndrome. Neurology(R) Neuroimmunology & Neuroinflammation, 9(1), e1106.
Stowe, A. M., Ireland, S. J., Ortega, S. B., Chen, D., Huebinger, R. M., Tarumi, T., Harris, T. S., Cullum, C. M., Rosenberg, R., Monson, N. L., & Zhang, R. (2017). Adaptive lymphocyte profiles correlate to brain Aβ burden in patients with mild cognitive impairment. Journal of Neuroinflammation, 14(1), 149.
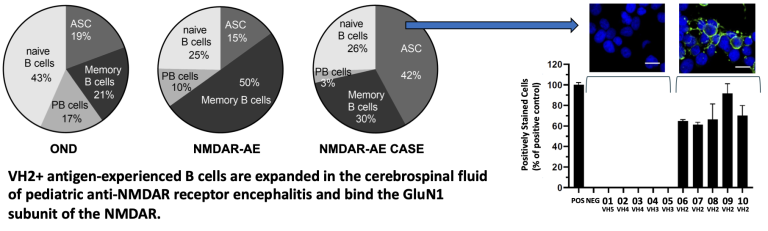
Autoimmune encephalitis caused by antibodies against the N-methyl-D-aspartate receptors (NMDARs) are the most common cause of antibody-mediated encephalitis in the world. NMDARs are critically involved in brain development, function and neurodegeneration. Patients with anti-NMDAR autoantibodies present with a diversity of symptoms including psychosis, hallucinations, personality changes, movements disorders and/or seizures. Clinical and radiographic studies have failed to identify the cause of this phenotypic variability. Detailed structural and functional characterization of human anti-NMDAR antibodies may be used to elucidate phenotypic variability. Our team has demonstrated that pediatric patients with anti-NMDAR autoimmune encephalitis have an expansion of antigen experienced B cells within the CSF that utilize antibody genes from the Variable Heavy Chain Family 2. Cloned antibodies from these B cells displayed a unique CDR3 motif that dictated binding to the GluN1 subunit of the NMDAR. Future studies will elucidate the binding domains of these antibodies.
Monson, N., Smith, C., Greenberg, H., Plumb, P., Guzman, A., Tse, K., Chen, D., Zhang, W., Morgan, M., Speed, H., Powell, C., Batra, S., Cowell, L., Christley, S., Vernino, S., Blackburn, K., & Greenberg, B. (2023). VH2+ Antigen-Experienced B Cells in the Cerebrospinal Fluid Are Expanded and Enriched in Pediatric Anti-NMDA Receptor Encephalitis. Journal of immunology (Baltimore, Md. : 1950), 211(9), 1332–1339.
McGetrick, M. E., Varughese, N. A., Miles, D. K., Wang, C. X., McCreary, M., Monson, N. L., & Greenberg, B. M. (2021). Clinical Features, Treatment Strategies, and Outcomes in Hospitalized Children With Immune-Mediated Encephalopathies. Pediatric Neurology, 116, 20–26.
Filatenkov, A., Richardson, T. E., Daoud, E., Johnson-Welch, S. F., Ramirez, D. M., Torrealba, J., Greenberg, B., Monson, N. L., & Rajaram, V. (2017). Persistence of parenchymal and perivascular T-cells in treatment-refractory anti-N-methyl-D-aspartate receptor encephalitis. Neuroreport, 28(14), 890–895.
We have partnered with Dr. Robert Haley on this project. Studies over the last two decades have provided evidence that symptoms associated with Gulf War Illness (GWI) may result from neurological, inflammatory and immune system dysfunction. However, there are no clinical tests available to diagnose GWI. Also, a lack of detailed understanding of the biological processes that cause disease has made it difficult to develop therapies for GWI. Recent advances in microarray proteomic and epigenetic technologies make possible the screening and identification of far more indicators of disease mechanisms than what was available to previous studies. The goal for this proposal is to apply the latest Next Generation technologies to the study of GWI. In particular, we are working to determine diagnostic GWI biomarker profiles that can also distinguish between distinct GWI syndrome variants.