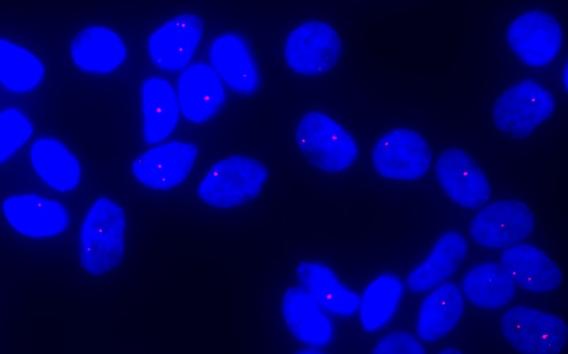
 Using Fluorescence In Situ Hybridization (FISH), expanded CUG repeat RNA molecules can be visualized as red dots in the corneal endothelial cells of patients with the TCF4 triplet repeat expansion.
Using Fluorescence In Situ Hybridization (FISH), expanded CUG repeat RNA molecules can be visualized as red dots in the corneal endothelial cells of patients with the TCF4 triplet repeat expansion. 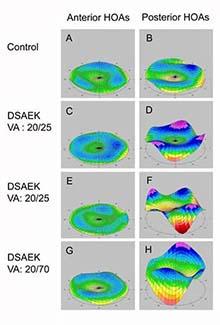 Legend: Rotating Scheimpflug imaging system (Pentacam) maps of corneal higher order aberrations (HOAs) of the central 6 mm (A) in a control eye (left: anterior corneal HOAs, right: posterior corneal HOAs), (B) in an eye that underwent penetrating keratoplasty (PKP), and in (C, D) 3 eyes that underwent Descemet stripping automated endothelial keratoplasy (DSAEK).
Legend: Rotating Scheimpflug imaging system (Pentacam) maps of corneal higher order aberrations (HOAs) of the central 6 mm (A) in a control eye (left: anterior corneal HOAs, right: posterior corneal HOAs), (B) in an eye that underwent penetrating keratoplasty (PKP), and in (C, D) 3 eyes that underwent Descemet stripping automated endothelial keratoplasy (DSAEK). Mootha Lab develops treatment options for corneal diseases and other eye disorders
We use human genetics and genomics to understand the molecular basis of Fuchs' endothelial corneal dystrophy and develop novel therapeutic strategies.
Research in the Mootha Lab currently encompasses two areas:
Genetics of Corneal Disorders
The long-term goal of my laboratory research program is to use human genetics and genomics to decipher the molecular basis of corneal endothelial disease and translate those basic discoveries back into the clinic.
Using both familial and trans-ethnic association studies, we have examined the strong association of intronic CTG triplet repeat expansions in the TCF4 gene with Fuchs’ endothelial corneal dystrophy (FECD). We reported that expanded CUG repeat-containing TCF4 transcripts form toxic nuclear foci in the corneal endothelial tissue of patients with the expansions. We observed that myotonic dystrophy type 1 patients with CTG expansions in DMPK gene are at increased risk for FECD and detected abundant CUG repeat foci in their endothelial cells. The most parsimonious explanation is that mutant CUG repeat RNA is causal for FECD disease. We showed that CUG repeat RNA is abundant in tissue from individuals who are pre-symptomatic and triggers changes in splicing and extracellular matrix gene expression which foreshadow the alterations observed in advanced disease.
In collaboration with the Corey Lab at UTSW, we have synthesized antisense oligonucleotides complementary to the CUG repeat and found them to be potent inhibitors of foci in cell culture and in human FECD tissue. We also demonstrated that repeat-targeting duplex RNAs and single-stranded silencing RNAs also reduce foci formation widening the potential for synthetic nucleic acids to be used to treat FECD. Additionally, we have demonstrated that the mutant CUG repeat RNA can also be effectively targeted using CRISPR and a catalytically dead Cas9.
Combining my clinical skills with my laboratory’s genetics expertise, including a group of expert collaborators, I lead studies aimed to examine the role of mutant repeat RNA in cellular disease, understand molecular pathway alterations observed early and late in the FECD disease course, and target the CUG repeat RNA for therapeutic development.
Emerging Techniques in Keratoplasty
I was amongst the first corneal transplant surgeons in Texas to offer Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) for patients with Fuchs’ endothelial corneal dystrophy. These endothelial keratoplasty procedures are less-invasive, better preserve the structural integrity of the globe, and offer more rapid visual recovery than full-thickness penetrating keratoplasty for patients with endothelial dysfunction.
Our research has focused on clinical outcomes and corneal remodeling after endothelial keratoplasty using the Department of Ophthalmology’s advanced anterior segment imaging capabilities including confocal microscopy, anterior segment optical coherence tomography, and Scheimpflug imaging.
Join Our Lab
Be part of the great impact we're having on science and medical care across the globe.The Mootha Lab is looking for highly motivated graduate students, postdocs, and technicians.