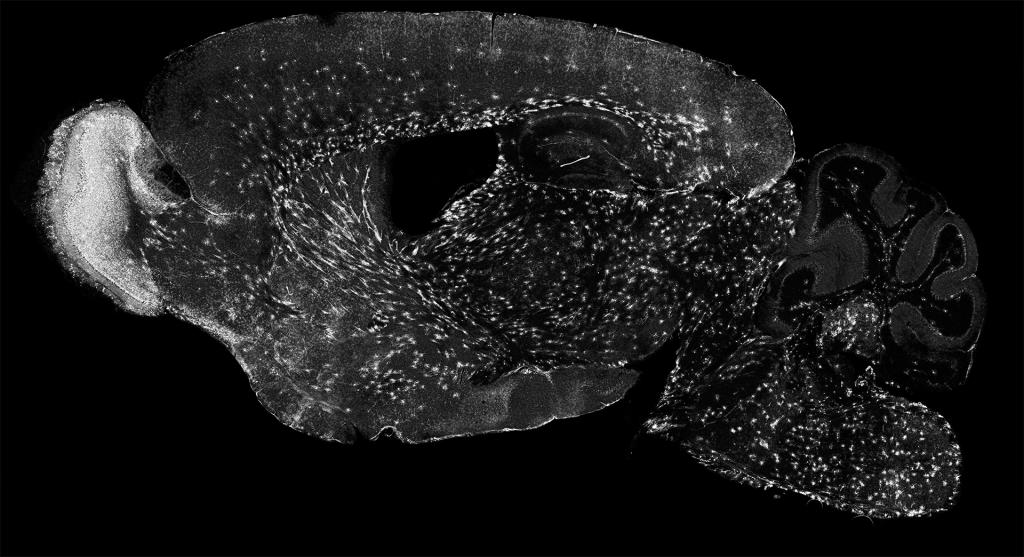
The Sun Lab studies the most numerous cells in the brain, called “glia”. Glia are essential for brain development, and glial abnormalities are associated with many neurological diseases, including cerebral palsy, stroke, autism, traumatic injury, multiple sclerosis (MS), and Alzheimer’s disease (AD). Dysregulation of glia often occurs prior to the onset of many neurological diseases, suggesting that they may drive disease occurrence and progression. However, the mechanisms underlying glial development and their communication with other cells remain poorly understood. We are dedicated to addressing the fundamental principles that govern glial formation and their communication with other cells in both health and disease.
In vertebrates (including humans), nearly half of the brain cells are non-neuronal cells. Most of them are glia, including oligodendrocytes, astrocytes, and microglia. Unlike neurons, glial cells do not fire action potentials, but they are essential for normal nervous system function. We have focused on oligodendrocytes, the sole myelin-producing cells in the central nervous system. Individual oligodendrocytes extend 3-80 processes (known as internodes) to myelinate nearby axons, thereby forming the myelin sheaths and providing critical insulation, trophic support, and antioxidant defense for axons. Because of their abundance, nearly half of the human brain’s dry mass is composed of oligodendrocytes and myelin. Despite their importance, how oligodendrocytes develop, myelinate axons, and become dysfunctional in diseases remains poorly understood.
Our lab is dedicated to addressing these questions with multidisciplinary approaches. We have combined mouse genetics, oligodendrocyte-neuron co-culture, volume electron microscopy, functional multiomics, and neurophysiology to investigate the underlying cellular and molecular mechanisms. Understanding how oligodendrocytes develop and communicate with other cells is essential to tackle many neurological diseases, including cerebral palsy, demyelinating diseases, glioblastoma, and dementia, that are significantly affected by oligodendrocyte and myelin dysfunction.
We are seeking highly motivated postdoctoral fellows and graduate students to join our team.
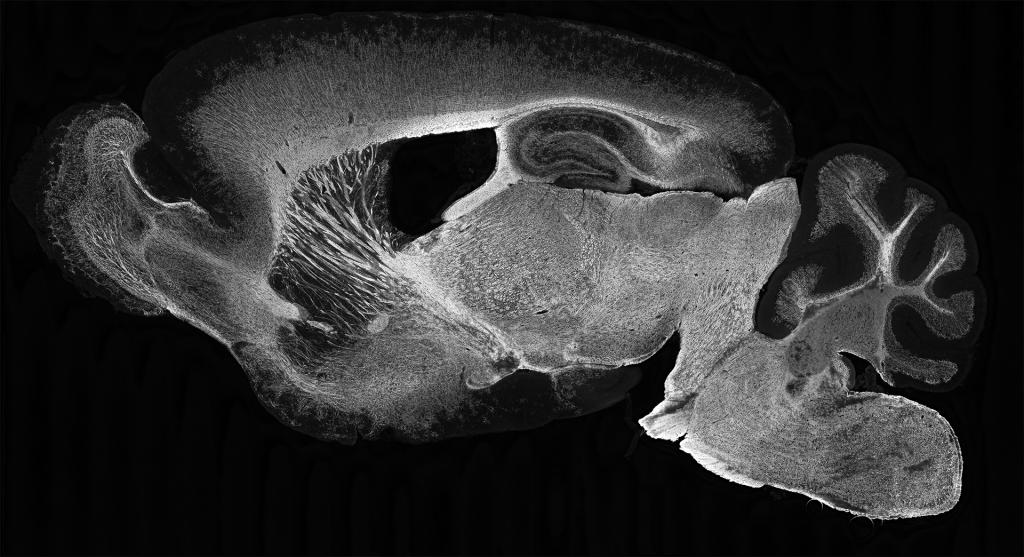
Our Research
Genetic decoding of the bottleneck in oligodendrogenesis
During both development and myelin repair, oligodendrocyte progenitor cells (OPCs) must exit the cell cycle and differentiate into premyelinating oligodendrocytes (preOLs) before myelinating axons. Despite being transient, preOLs are at a critical stage of myelination, known as axon ensheathment. At this stage, they extend several processes to survey and begin ensheathing nearby axons. Due to their critical roles in oligodendrogenesis, dysregulation of preOL survival and maturation is evident in multiple sclerosis and perinatal white matter injury. The lack of genetic tools to visualize and manipulate preOLs has left this critical differentiation stage woefully understudied. Using the Easi-CRISPR, we have engineered a CreERT2 knock-in mouse line that allows genetic labeling, lineage tracing, manipulation, and multimodal profiling of preOL subsets across the central nervous system (Bhambri et al., 2024 BioRxiv; Nature Neuroscience, accepted). Our work provides a new tool to probe this critical cell stage during development, allowing for the investigation of myelin establishment.
Genome-wide CRISPR knockout screens to identify new players in oligodendrogenesis
To uncover the intrinsic mechanisms governing CNS myelination, we have developed a primary oligodendrocyte-based, genome-wide CRISPR knockout screen platform that enables us to identify several new pathways controlling oligodendrogenesis. Specifically, we established a new method to purify primary murine oligodendrocyte precursor cells and expand them in large quantities sufficient for genome-wide CRISPR screens. Our method ensures that these proliferating oligodendrocyte progenitor cells maintain essential properties of their in vivo counterparts and display stereotypical differentiation in culture. Using this system, we have identified several pathways that have been poorly studied in myelination. We are developing an in vivo CRISPR screen to fully understand the intrinsic and extrinsic mechanisms underlying myelination.
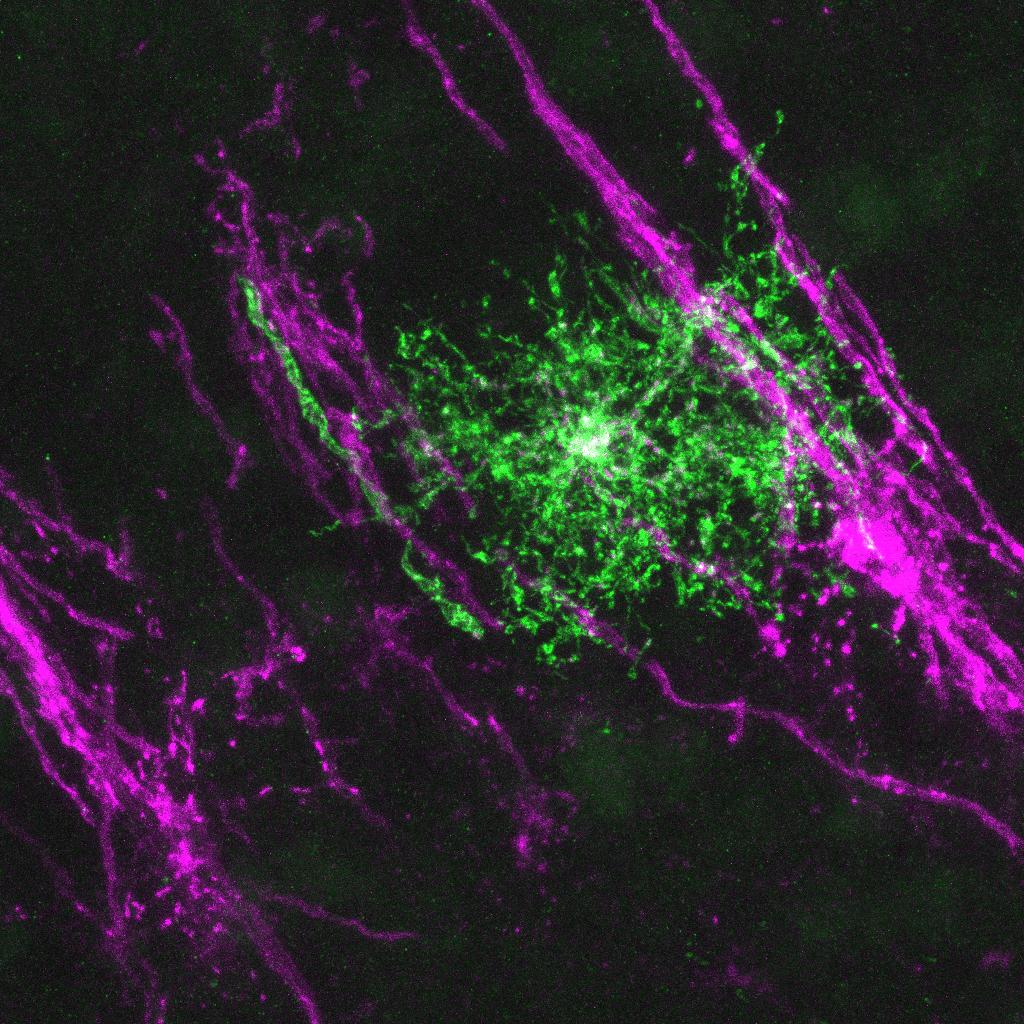
Molecular mechanisms underlying axon-oligodendrocyte communications
Axons play instructive roles in determining the spatiotemporal specificity and the levels of myelination. It remains largely unclear how extrinsic neuronal signals coordinate with the oligodendroglial intrinsic program to achieve proper myelination levels. Our recent work discovered a missing molecular link connecting CNS axons with oligodendrocytes. Using mouse genetics, we found that CNS axons secrete cues to powerfully induce a nucleus-to-cytoplasm translocation and subsequent inactivation of an oligodendrocyte-enriched transcription factor (TFEB). Surprisingly, TFEB directly binds to numerous cholesterol biosynthesis genes to reduce cholesterol levels, thereby inhibiting myelination when when it is in the nucleus of oligodendrocytes (Zhang et al., 2025 Cell Reports). The identity of neuron-derived factor(s) remains unknown. Moreover, it is of great interest to test whether oligodendroglial TFEB can function as a sensor to detect axon damage.
Cellular and molecular mechanisms that suppresses central nervous system myelination
Hypomyelination, such as a reduction in myelin sheath thickness, often leads to severe brain dysfunction in multiple sclerosis and other neurological disorders. Conversely, the mechanisms that naturally suppress myelination remain poorly understood. These mechanisms could be harnessed to enhance remyelination in MS. Our previous work has identified a key molecular pathway that potently represses CNS myelination (Sun et al., Cell 2018). Recently, we have identified a parallel pathway, orchestrated by autophagy, that inhibits CNS myelination (Zhang et al., Cell Reports 2023). Our ongoing work has addressed the downstream mechanisms of autophagy and discovered an unexpected role of the endoplasmic reticulum (ER) in controlling lipid biosynthesis and myelin sheath growth.
Mechanisms governing myelin integrity during axon ensheathment and re-ensheathment
Myelin sheath formation has long been considered an almost flawless process, resulting in a smooth, tight wrapping of axons. However, recent studies suggest that myelin abnormalities, such as myelin whorls, outfoldings, and myelin-on-myelin structures, arise naturally during development and are gradually resolved over time. Among them, myelin whorls or myelinosomes, characterized by multi-layered degenerative myelin, are a major myelin abnormality frequently found in human demyelinating diseases prior to oligodendrocyte process degeneration and cell loss. How myelin whorls are formed and eliminated remains poorly understood. Our recent work identifies a quality control system that functions intrinsically within oligodendrocytes to safeguard myelin integrity (Barbosa et al., under review). Our ongoing work aims to determine the extent to which this system functions in demyelination, and whether it can be harnessed to ensure myelin integrity during remyelination.
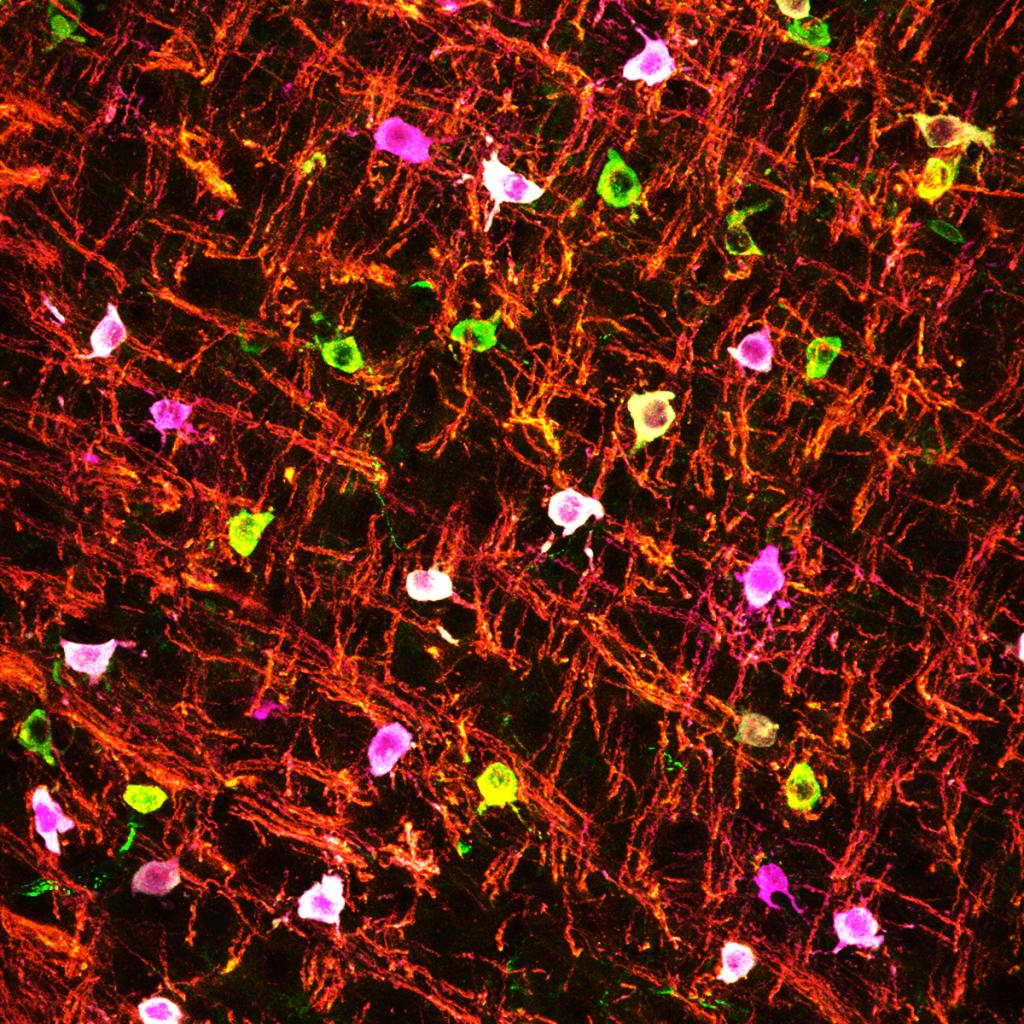
Oligodendrocytes are involved in numerous diseases, including cerebral palsy, psychiatric diseases, multiple sclerosis, glioblastoma, and dementia. We have focused on understanding why immature oligodendrocytes are extremely vulnerable in diseases such as neonatal white matter injury, as well as the mysterious “disease-associated oligodendrocytes” in neurological disorders. We are currently developing new genetic and viral tools to begin addressing these questions.

CNS axons are thought to be heavily myelinated. Recent findings, however, show the opposite. For instance, many CNS axons are intermittently myelinated with numerous unmyelinated segments spaced in between myelinated segments, possibly allowing neural activity-dependent myelination. To begin to map and manipulate CNS myelination, we have generated and characterized several novel knock-in mouse strains that label distinct oligodendrocyte differentiation stages including premyelinating oligodendrocytes (Enpp6-IRES-CreERT2; Bhambri et al., 2024) and myelinating oligodendrocytes (Mog-IRES-Cre and Mog-IRES-CreERT2; Zhang et al., 2025). We are developing both viral and genetic tools to map and manipulate myelination in an axon-specific manner.