General Research Overview
Inflammation is a risk factor for several types of cancers. In the case of colitis associated cancer (CAC), ulcerative colitis (UC), a disease of that involves the chronic inflammation of the colon, has a risk factor with a 3-5-fold increase for developing sporadic colorectal cancer (CRC) than does the general population. In CRC, the fibroblasts of the mesenchymal inflammatory microenvironment contribute to CRC pathogenesis. In CAC, such a relationship is less clear with microenvironmental genotoxic contributions from aberrant p53 and reactive oxygen species preceding oncogenesis. Currently, there are no curative, CAC-preventing, or CAC-reversing options for UC patients, as the pathogenesis remains unclear. Due to this unknown pathogenesis, our long-term goal is to understand the pathogenesis of UC and CAC, so that the development of preventative measures becomes more feasible.
 Colorectal Cancer
Colorectal Cancer Colorectal Cancer Metastases
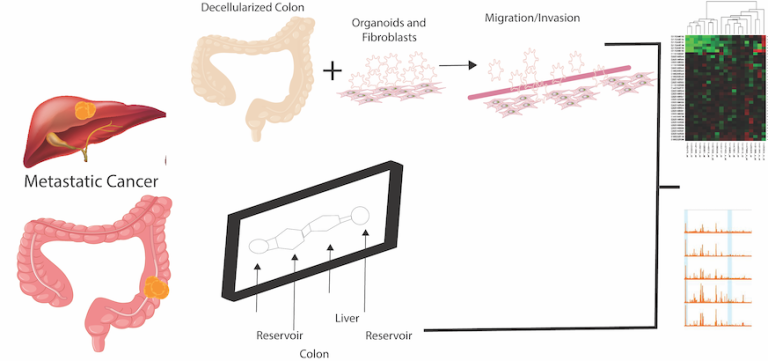
Patients who develop colorectal cancer metastases have a poor prognosis. In our laboratory, we are developing tissue-engineered models, including models for in vitro intravasation, and organoids to model these diseases. Dr. Huang is a member of the Tissue Engineering Collaborative of the National Cancer Institute. Her team includes another principal investigator, Xiling Shen, PhD, Duke, who is a biomedical engineer, and co-investigator, Michael Shuler, PhD, Cornell, who is also a biomedical engineer. Together, they have derived numerous in vitro and in vivo models.
Early-Onset Colorectal Cancer (EO CRC)
The number of patients < 50 years of age with colon cancer and rectal cancer is expected to double and quadruple by 2030. The etiology is unknown, but may be due to influences of metabolism and inflammation. With contemporary tools and models, the Huang lab plans to define mechanisms and approaches to understand and to prevent EO CRC.
Current Research Summaries
Current treatments of metastatic colorectal cancer (CRC) have only limited efficacy, and research is hindered by a lack of adequate models, ones that are technically feasible and that accurately recapitulate human metastasis (invasion, metastasis, and the effects of the microenvironment [the extracellular matrix; immune cells]). Thus, we are looking to develop and test better metastatic models.
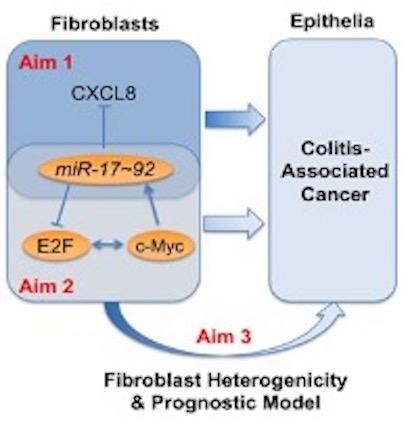
We recently reported that colitis-associated fibroblasts and cancer-associated fibroblasts from UC patients secrete elevated levels of CXCL8 (an inflammatory mediator), that CXCL8 levels are negatively regulated by a miRNA, miR-20a. Furthermore, miR-20a is at least in part responsible for the modulation of CXCL8 secretion in interstitial fibroblasts. miR-20a is known to be modulated by c-Myc via interaction with the E2F family of transcription factors. However, in contrast to colon epithelia data, our data indicates that low miR-20a and low c-Myc levels in colitic fibroblasts increase tumorigenicity. To resolve this conundrum, the overall objective of this application, is to determine the contribution of fibroblast miR-20, a member of the miR-17 family, to the pathogenesis of CAC.