During early embryogenesis in mammals, LIN28 paralogs regulate the pluripotency of embryonic stem cells by binding to and inhibiting members of the let-7 family of microRNAs, while at later stages let-7 binds to and suppresses LIN28A/LIN28B mRNAs.
This complex feedback system, only partially understood, is also hypothesized to regulate stem-cell-like cancer cells. The plasticity of cancer stem cells (CSCs) drives the heterogeneity of tumor cells with different metastatic potential, immune resistance, or resistance to chemotherapy and targeted therapy.
Our previous work has uncovered interactions with MYC (MYC Proto-Oncogene, BHLH Transcription Factor), as well as Sox2 and Sox9, which causes tumors to convert from proliferation to metastasis.
We anticipate that further studies of binding mechanics and timing in this complex system of LIN28/let-7-mediated plasticity, in normal embryogenesis and in cancerous conditions, will lead to rational develop of therapies against chemoresistance and metastasis.
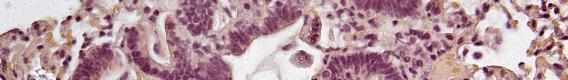
Migration of HCC2352 squamous lung cancer cells in an in vitro model of wound healing, which shares many characteristics with metastatis. Loss of green staining indicates biochemical changes currently under study (unpublished data).