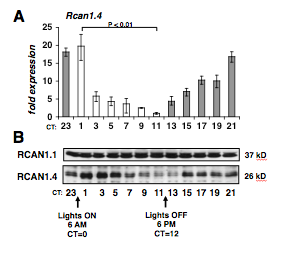
Many important cardiovascular factors oscillate over a 24-hour period. In humans, the incidence of adverse cardiac events vary according to the time of day, and forced changes in circadian rhythm are associated with increased risk for heart failure. We have recently found evidence of large circadian oscillations in RCAN1.4 transcripts, RCAN1.4 protein levels and calcineurin activity in normal, healthy hearts. This finding is remarkable because activation of calcineurin has primarily been thought of as a pathological process driving cardiac hypertrophy and failure.
Studies are designed to identify the processes controlled by these oscillations and how they help coordinate changes in cardiac unction and remodeling in anticipation of changes in physiological demand. Both human and rodent hearts are more susceptible to damage from ischemia-reperfusion at the time of waking. This corresponds to the time of day when RCAN1.4 levels are lowest in the heart. Remarkably, we have found that mice lacking the Rcan1 gene display maximal damage regardless of the time of day suggesting that RCAN1 mediates circadian changes in the susceptibility of the heart to damage.
Rcan1.4 expression is circadian in all tissues we have tested, and mice lacking Rcan1 have a longer innate circadian day length. We hypothesize that calcineurin/RCAN1.4 oscillations are a fundamental point of cross-talk between circadian oscillations in cytoplasmic calcium levels and the transcriptional clock mechanism.