In the Wu lab, the research focuses on how chronic infection, cancer and aging affect T-cell immunity. T cells are an important part of our immune system protecting us against pathogens and malignancies. However, T cell become dysfunctional or exhausted, during chronic infection and cancer.
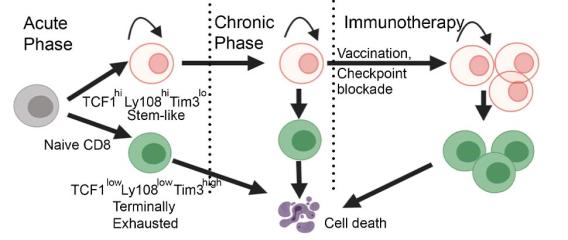
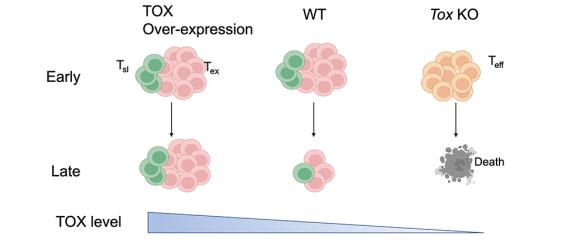
One of the directions in the lab is to understand the molecular and cellular mechanisms underlying T-cell exhaustion and to identify ways to reinvigorate T-cell immunity against chronic infection and cancer. We recently found that T cells are not equally exhausted in chronic infection or cancer. A subset of T cells, termed stem-like T cells, persist and replenish other exhausted T cells. Importantly, stem-like T cells are required for the efficacy of many types of immunotherapies including immune checkpoint blockade.
Using single-cell multiomics and CRISPR/Cas-mediated genome editing, we have illustrated the transcription factors that induce the molecular program of stem-like T cells and distinguish them from other exhausted T cells. We are also interested in the molecular circuit that governs the differentiation of stem-like T cells into different progenies. Besides transcriptional regulation, we are working to understand the metabolic pathways that give stem-like T cells the ability to mount a proliferative burst upon immune checkpoint blockade. In addition, we are using tissues from cancer patients to identify pathways in T cells that determine the antitumor efficacy and/or immune-related adverse events during immunotherapies.
T-cell dysfunction also occurs during aging. Immunosenescence of T cells increases morbidity and mortality after infection and reduces vaccine efficacy. Using in vitro and in vivo models, we have found that aging impairs the metabolic adaptation and effector program of T cells after activation. We are currently dissecting the molecular mechanism responsible for these changes.