When I became a postdoctoral fellow, I switched my research focus from insects to rodents. I began measuring electrical activity, intracellular calcium, ATP, cAMP, and neuropeptides from the pacemaker in the suprachiasmatic nucleus (SCN).
I became interested in how individual SCN neurons are coupled to drive robust circadian locomotor activity, and how other neuronal circuits are involved in this process.
Measuring multi-unit activity from freely behaving animals provided evidence that locomotor activity feeds back and alters the neural activity in the SCN.
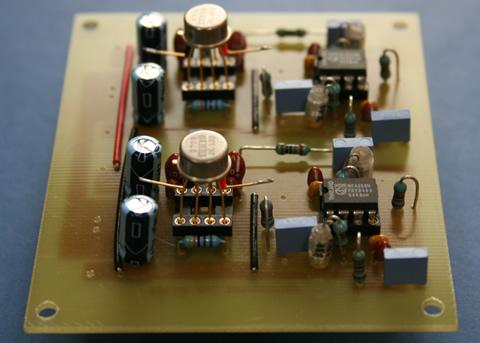 Differential amplifier specifically designed for multi-unit activity (MUA) recording. Researchers at Osaka University, Teikyo Heisei University, Leiden University Medical Center, Kent State University are currently using this amp with field effect transistor input buffer head stage voltage follower to record MUA from freely behaving mice and rats.
Differential amplifier specifically designed for multi-unit activity (MUA) recording. Researchers at Osaka University, Teikyo Heisei University, Leiden University Medical Center, Kent State University are currently using this amp with field effect transistor input buffer head stage voltage follower to record MUA from freely behaving mice and rats. Peripheral circadian clocks: a paradigm shift
During my postdoctoral work, the circadian field transformed rapidly, as researchers cloned circadian genes in both Drosophila and mammals.
In collaboration with Hajime Tei, Ph.D., who successfully cloned a mammalian homologue of the Drosophila period gene, we developed a rodent model for monitoring real-time circadian gene expression rhythm by bioluminescence.
Using this technique, we discovered circadian clocks in mammalian peripheral organs. As a result, we view the mammalian circadian system as a multi-oscillator system, rather than a system controlled by one pacemaker structure in the SCN.
The discovery also allows biochemical analysis to be performed in abundant tissues such as the liver, which accelerated understanding of the molecular mechanisms of timekeeping.
We realized that out-of-sync relationships among clocks in peripheral organs contribute to many human diseases. Every tissue's internal circadian rhythm delicately controls the local physiology of that tissue; even small changes in that timing can result in malaise, such as metabolic syndrome and obesity.
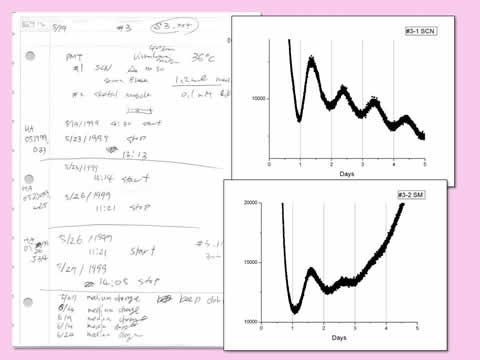 May 19, 1999: Discovery of an endogenous clock in a peripheral organ. Both SCN and skeletal muscle explanted from a Per1-luc transgenic rat showed luminescence rhythms in culture. Work performed in Menaker/Block labs at the University of Virginia, witnessed by Michikazu Abe and Shin Yamazaki.
May 19, 1999: Discovery of an endogenous clock in a peripheral organ. Both SCN and skeletal muscle explanted from a Per1-luc transgenic rat showed luminescence rhythms in culture. Work performed in Menaker/Block labs at the University of Virginia, witnessed by Michikazu Abe and Shin Yamazaki.  Original PMT system used for luminescence recording. The original setup was only two channel; we added more recording channels after successful recording of luminescence rhythms from the SCN and many other peripheral tissues.
Original PMT system used for luminescence recording. The original setup was only two channel; we added more recording channels after successful recording of luminescence rhythms from the SCN and many other peripheral tissues. Dysregulation and disease: Cause or effect?
To investigate circadian organization, our group developed a method for generating phase maps by measuring circadian gene bioluminescence in acute tissue explants.
Using this technique, we have shown how the system changes during jet lag and shift work. We also investigated how circadian organization changes during ontogeny and aging and identified tissues whose phases are shifted by timed restricted feeding or high-fat diet.
From these studies, we have learned that the multi-oscillatory circadian system is carefully tuned for adaptation to changes in the environment.
I believe that the continuation of our studies investigating the factors that impact circadian organization will provide strategies for preventing diseases as well as novel therapeutics. But we must be cautious to distinguish between whether circadian disruption causes or is a consequence of disease.
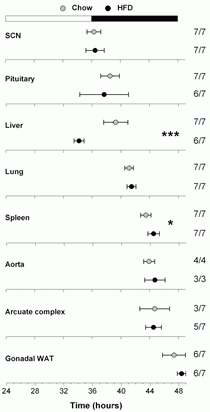 Phase map of mice fed 45 percent high-fat diet for one week. The phase of the liver was advanced by five hours, while the phases of the other tissues were not affected by consumption of high-fat diet. Figure from Pendergast et al. (2013), High-fat diet acutely affects circadian organization and eating behavior. European Journal of Neuroscience 37(8):1350-6. (Reproduced with permission.)
Phase map of mice fed 45 percent high-fat diet for one week. The phase of the liver was advanced by five hours, while the phases of the other tissues were not affected by consumption of high-fat diet. Figure from Pendergast et al. (2013), High-fat diet acutely affects circadian organization and eating behavior. European Journal of Neuroscience 37(8):1350-6. (Reproduced with permission.)