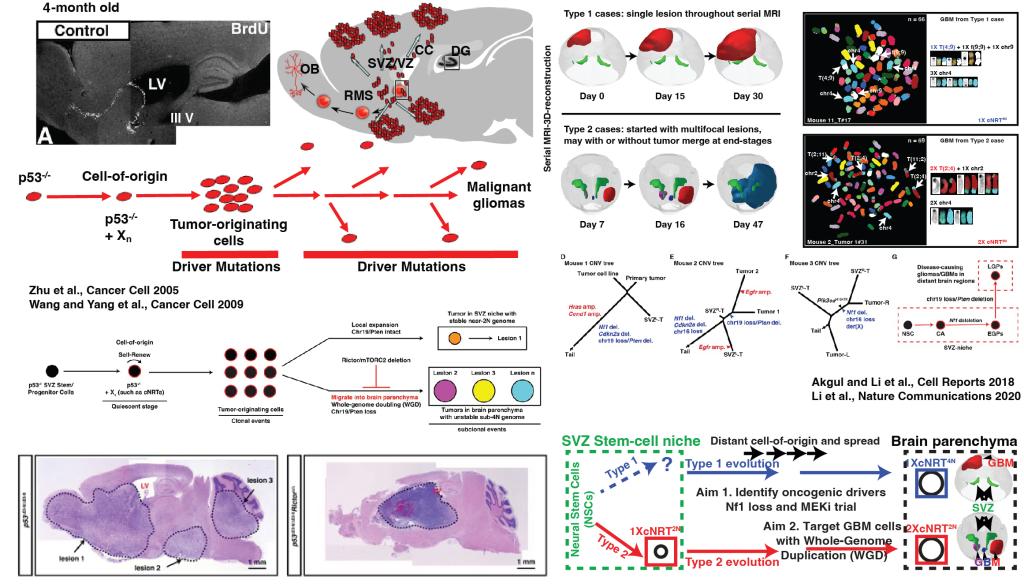
GBM is the most common primary brain tumor in adults with no pre-existing lower-grade lesions. The rapid clinical course of adult GBMs presents a major challenge to develop a preventive therapy. A recent study revealed spatial segregation of tumor initiation and manifestation in the subventricular zone (SVZ) stem-cell niche and brain parenchyma, providing an explanation for adult GBMs with clinically undetected, but evolutionarily inferred, early tumor progenitor cells. Using serial MRI/3D-reconstruction, whole-genome sequencing, and SKY (spectral karyotyping)-based single-cell phylogenetic tree building, we demonstrated that a series of p53-mutated mouse GBM models recapitulate this unique evolutionary pattern of spatial segregation of tumor initiation and manifestation, providing a preclinical platform to investigate and target the distant spread of GBM in the brain. Using these p53 mutant-driven single stem-cell-derived GBM models, we will investigate (1) canonical versus non-canonical p53-mediated tumor suppressive functions in quiescent and active neural stem cells, (2) determine molecular mechanisms underlying local expansion and the distant spread of GBM cells from the SVZ to brain parenchyma, and (3) develop novel therapeutic strategies for adult GBMs with a focus on the identification of drugs to specifically kill cancer cells with whole-genome duplication. While it is currently challenging to develop a clinical trial to prevent adults with GBMs, a recent study showed that half of gliomas arising from Li-Fraumeni syndrome (LFS) patients carried genetic alterations in both TP53 and NF1. There is a potential to run a parallel preclinical and clinical preventive trial using our mouse GBM model and LFS patients.
Pediatric Low-Grade Glioma (pLGG) and High-Grade Brain Tumor