May 2025
Elizabeth has been elected to serve as President of the American Society for Cell Biology (ASCB) in 2027.
ASCBApril 2025
Danqing Dong received the Best Talk Award at the Cell and Molecular Biology (CMB) graduate program retreat.
March 2025
Yue Lu received an American Heart Association (AHA) Career Development Award.
February 2025
Benjamin Ravaux gave a platform talk at the Biophysical Society meeting.
February 2025
Merging of Muscle Cells
Yue Lu’s live imaging video of myoblast fusion has been featured on HHMI Beautiful Biology.
Merging of Muscle CellsJanuary 2025
Researchers unlock key step in cell fusion process
Our work on how cells generate invasive protrusions to promote cell fusion (Lu et al., Nat. Cell Biol. 2024) is featured by UT Southwestern CenterTimes Plus.
CenterTimes PlusJanuary 2025
Paper published!
Our paper demonstrating a role for branched actin polymerization in promoting myoblast fusion during muscle regeneration is published: Lu et al., eLife.
eLifeDecember 2024
Yue Lu was selected for a Best Poster Award at the Cold Spring Harbor Laboratory meeting on Cell & Membrane Fusion.
November 2024
Our paper on the molecular regulation of invasive protrusions at the mammalian fusogenic synapse is published: Lu et al., Nat. Cell Biol. Online ahead of print.
October 2024
Danqing Tong received a travel award from the UT Southwestern Graduate Student Organization (GSO).
August 2024
Benjamin Ravaux won the top prize of the 2023-2024 CRSM Postdoc WIPS awards.
March 2024
Our paper elucidating the role of ecdysone signaling in myoblast fusion is published: Ruan et al., Curr Biol. Online ahead of print.
May 2023
Amrita Gokhale defended her thesis.
November 2022
Lab alum Jun Shi (2022) took a faculty position at South China Agricultural University.
November 2022
Tezin Walji defended her thesis.
September 2022
Elizabeth Chen recognized as a 2022 American Society for Cell Biology (ASCB) fellow.
May 2022
Lab alum Ruihui Zhang (2022) took a faculty position at Huazhong Agricultural University.
June 2022
Our paper identifying the cellular architecture and molecular determinants of the zebrafish fusogenic synapse is published: Luo et al., Dev. Cell Online ahead of print.
Aug 2020
Tezin Walji received a diversity supplement award from the National Institute of Health.
June 2020
Our paper on Dynamin's regulation of the dynamics and mechanical strength of the actin cytoskeleton.
Recommended by Faculty Opinions: Paolo Provenzano, Oliver Daumke, and Ling-Gang Wu.
View Article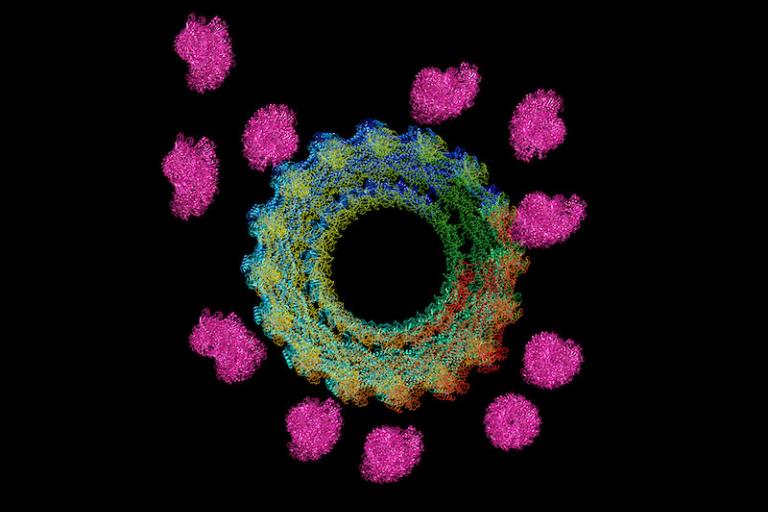
May 2020
Urge to merge: Understanding how cells fuse.
DALLAS – May 25, 2020 – Scientists have known for a decade that cells that fuse with others to perform their essential functions – such as muscle cells that join together to make fibers – form long projections that invade the territory of their fusion partners. But how the thin and floppy polymers involved in this process propel mechanically stiff protrusions has been unknown.
In a new study published online today in Nature Cell Biology, UT Southwestern scientists outline the mechanisms behind the formation of these projections, focusing on the interaction between two proteins known as actin and dynamin. The findings, they say, offer insight into a key cellular process that’s essential for the conception, development, regeneration, and physiology of multicellular organisms and may eventually lead to new treatments for a rare muscle disease.
May 2020
Our paper on Dynamin's regulation of the dynamics and mechanical strength of the actin cytoskeleton is published: Zhang et al., Nat. Cell Biol. 22,674-688.
January 2020
Benjamin Ravaux received a travel award from the Wellstone Muscular Dystrophy Center.
July 2019
Our review on Drosophila myoblast fusion is published: Lee and Chen, Annu. Rev. Genet. 53, 67-91.
July 2019
Jun Shi received a travel award from the 14th International Zebrafish Conference.
April 2019
Santosh Verma took a faculty position at Sanjay Gandhi Postgraduate Institute of Medical Sciences, India.
November 2018
Ruihui Zhang was awarded an American Heart Association Postdoctoral Fellowship.
October 2018
Ruihui Zhang received the Wellstone Center Travel Award.
July 2018
Elizabeth received the American Society for Cell Biology WICB Mid-Career Award for Excellence in Research.
CenterTimes PlusJuly 2018
Nathalie Gerassimov defended her thesis.
June 2018
Jun Shi received a 13th International Zebrafish Conference travel award.
May 2018
Our paper on the mechanoresponsive protein Spectrin is published: Duan et al., Nat. Cell Biol. 20, 688-698.
October 2017
Our first zebrafish paper is published: Shi et al., Proc. Natl. Acad. Sci. U.S.A. 144, 11950-11955.
April 2017
Rui Duan started his faculty position at South China Normal University.
July 2016
Donghoon Lee received a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR).
June 2016
Elizabeth received a Faculty Scholar Award from HHMI.
June 2016
We moved our lab from Johns Hopkins to UT Southwestern.
June 2016
Nathalie Gerassimov received a predoctoral fellowship from the American Heart Association (AHA).
June 2015
Khurts Shilagardi received a National Scientist Development Grant from the American Heart Association (AHA).
April 2015
Ji Hoon Kim received the Daniel Nathans Award of the Young Investigator’s Day Program of Johns Hopkins University.
April 2015
Cellular ‘Cruise Control’ Systems Let Cells Sense and Adapt to Changing Demands.
Cells are the ultimate smart material. They can sense the demands being placed on them during critical life processes and then respond by strengthening, remodeling, or self-repairing, for instance. To do this, cells use “mechanosensory” systems similar to the cruise control that lets a car’s engine adjust its power output when going up or down hills.
Researchers are uncovering new details on cells’ molecular cruise control systems. By learning more about the inner workings of these systems, scientists hope ultimately to devise ways to tinker with them for therapeutic purposes.
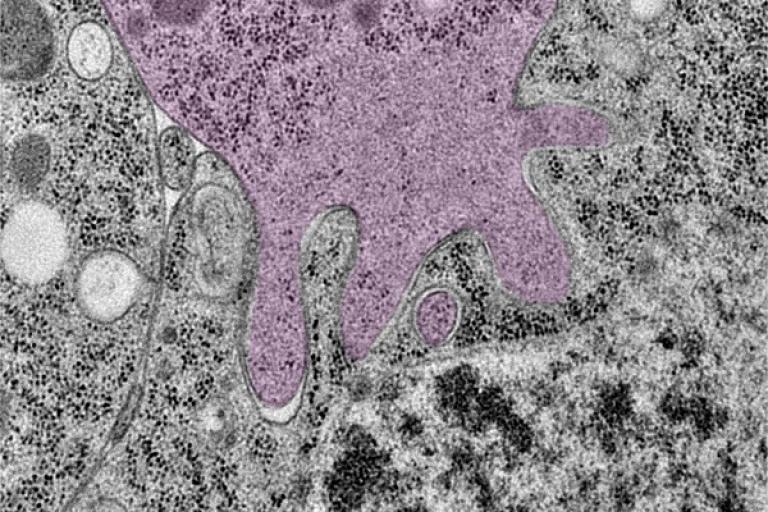
February 2015
Mechanical stress is a key driver of cell-cell fusion, study finds.
Just as human relationships are a two-way street, fusion between cells requires two active partners: one to send protrusions into its neighbor, and one to hold its ground and help complete the process. Researchers have now found that one way the receiving cell plays its role is by having a key structural protein come running in response to pressure on the cell membrane, rather than waiting for chemical signals to tell it that it's needed. The study helps open the curtain on a process relevant to muscle formation and regeneration, fertilization, and immune response.
View Article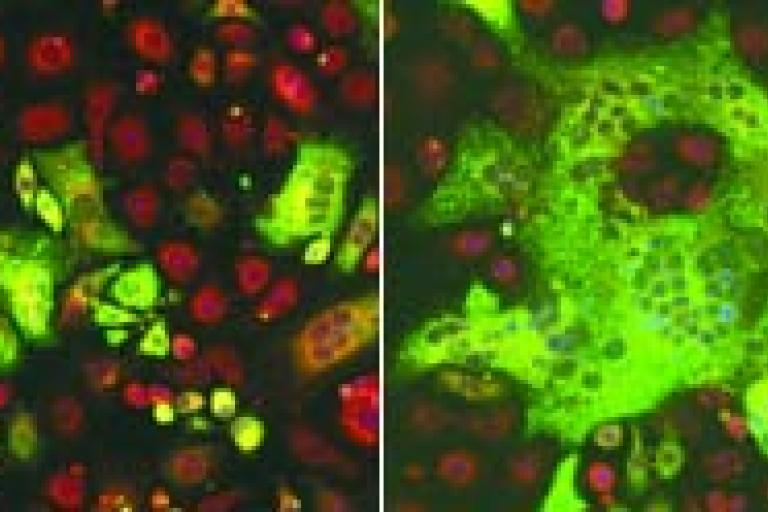
March 2013
Johns Hopkins study provides key insight into how cells fuse.
Researchers at Johns Hopkins have established a high-efficiency cell-cell fusion system, providing a new model to study how fusion works. The scientists showed that fusion between two cells is not equal and mutual as some assumed, but, rather, is initiated and driven by one of the fusion partners. The discovery, they say, could lead to improved treatments for muscular dystrophy, since muscle regeneration relies on cell fusion to make muscle fibers that contain hundreds or even thousands of nuclei.
The study, published online on Mar. 7 in Science, reveals two critical components that have to be present for cell fusion to happen, explains Elizabeth Chen, Ph.D., an associate professor of molecular biology and genetics at the Johns Hopkins University Institute for Basic Biomedical Sciences. Intriguingly, she says, one of these vital components changes the structure of one cell’s scaffolding — its cytoskeleton — to form protrusions that push their way into the other cell to initiate fusion.
July 2012
Rui Duan was awarded a postdoctoral fellowship from the American Heart Association (AHA).
July 2008
Khurts Shilagardi was awarded a postdoctoral fellowship from the American Heart Association (AHA).
July 2008