Cell-cell fusion is an indispensable cellular process in the development and physiology of multicellular organisms. It is required for fertilization, skeletal muscle development, bone resorption, immune response, placental formation, and stem cell-mediated tissue regeneration. Failure in cell-cell fusion may lead to a plethora of defects such as infertility, congenital myopathy, osteopetrosis, immune deficiency, and preterm birth. Despite the diversity of cell types that undergo fusion, all cell-cell fusion events start with the recognition and adhesion of two fusion partners and end with plasma membrane merger and exchange of cytoplasmic material. We are interested in uncovering the general mechanisms underlying cell-cell fusion and understanding its dysregulation in human diseases.
During skeletal muscle development, mononucleated myoblasts fuse to form multinucleated, contractile muscle fibers. Myoblast fusion is an evolutionarily conserved process that occurs in organisms ranging from insects to human. We are using three different experimental models, Drosophila, zebrafish and mouse, to study mechanisms of myoblast fusion. Leveraging the powerful genetic tools in Drosophila, we have conducted unbiased genetic screens to identify genes required for myoblast fusion and characterized their functions. Taking advantage of transparent zebrafish embryos that develop outside the mother, we have visualized the cellular structure mediating myoblast fusion in a live vertebrate animal. Using mouse as a mammalian model, we are studying myoblast fusion in skeletal muscle development, regeneration and disease.
Our genetic and cell biological studies led to the discovery of an actin-enriched podosome-like structure (PLS) at the site of Drosophila myoblast fusion. The PLS is only generated in one of the two fusing partners (invading cell) and projects finger-like protrusions into the receiving cell to increase membrane contact area and facilitate membrane juxtaposition and fusion. The unilateral PLS invasion conforms a cellular asymmetry at the site of fusion, which we termed the fusogenic synapse. Similar invasive structures have been observed during the fusion of both muscle and non-muscle cells in Drosophila, zebrafish and mammals, making this a conserved and general mechanism underlying cell-cell fusion.
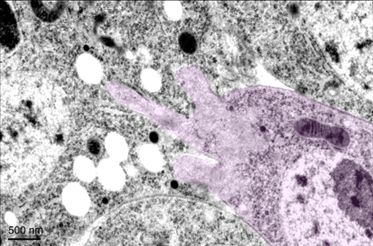
Invasive protrusions are generated by Arp2/3-mediated actin polymerization at the fusogenic synapse. Genetic screens identified essential functions for the actin nucleation-promoting factors of the Arp2/3 complex, WASP and Scar, in myoblast fusion. In addition, our molecular, cell biological, biochemical, biophysical, and cryo-ET studies revealed novel mechanisms underlying the regulation of the actin cytoskeleton. In particular, we identified a PH domain-containing protein blown fuse in destabilizing the WASP-WIP complex and modulating the actin cytoskeletal dynamics; the p21-activated kinase that acts downstream of Rac to organize the actin filaments within the PLS; the large GTPase dynamin that functions as a multi-filament actin-bundling protein in enhancing the mechanical strength of the actin network to generate invasive protrusions.
Using both Drosophila myoblast fusion and our reconstituted cell-fusion culture system, we discovered mechanosensory responses in the receiving fusion partner. Both the actin motor Myosin II and the membrane skeleton protein Spectrin exhibit mechanoresponsive accumulations at the fusogenic synapse in the receiving cell. Our genetic, cell biological, biophysical and mathematical modelling analyses demonstrate that the accumulated Myosin II increases the cortical stiffness in the receiving cell to resist the invasive forces, whereas the accumulated uneven Spectrin network functions as a cellular fence to restrict the cell adhesion molecules and a cellular sieve to constrict the invasive protrusions, thereby increasing the mechanical tension of the fusogenic synapse. The high mechanical tension, in turn, helps to overcome the energy barriers for membrane apposition and drives cell membrane juxtaposition and fusion.
Join Our Lab
Be part of the great impact we're having on science and medical care across the globe.Positions are available for highly motivated and creative postdoctoral fellows and graduate students who are interested in cell-cell fusion, muscle development, actin cytoskeleton, mechanobiology, and membrane biology.