Choi (Jaehyuk) Lab
Pioneering therapies for immune-related diseases.
The goal of the Choi Lab is to develop novel immunotherapies. To accomplish this, we first utilize high-dimensional approaches on human disease samples. This approach identifies targetable disease-promoting molecular defects in immune cells. Then, we utiize engineering approaches to reverse molecular changes.

Dr. Choi the inaugural Director of the new Center for Cellular Therapies and Cancer Immunology in the Harold C. Simmons Comprehensive Cancer Center. He also serves as Vice Chair for Translational Research and Innovation in the Department of Dermatology and holds the Scheryle Simmons Patigian Distinguished Chair in Cancer Immunobiology. After completing undergraduate studies in biochemistry at Harvard University, Dr. Choi earned his medical degree and Ph.D. in immunobiology from Yale University School of Medicine. He has been recognized with a number of awards, including the Damon Runyon Clinical Investigator Award (2016), the NIH Director’s New Innovator Award (2017), and the Mark Foundation Emerging Leader Award (2022).
An imbalance of T cells, which play a key role in the body’s immune response, could explain most cases of the disease, according to new research.
Jaehyuk Choi, M.D., Ph.D., a physician-scientist whose discoveries have transformed the field of cancer immunology, has begun his new role as the inaugural Director of the Center for Cellular Therapies and Cancer Immunology in the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern. He also serves as Vice Chair for Translational Research and Innovation in the Department of Dermatology.
For some people with blood cancers like leukemia and lymphoma, CAR T-cell therapies have proven to be a transformative treatment. But for solid tumors like breast, colorectal, or pancreatic cancer, which make up about 90% of cancer cases, success with T-cell therapies has been harder to come by.
T cell therapies have revolutionized cancer therapy for haematological cancers but have not yet been consistently effective in solid tumours, which represent 90% of adult cancers3. In both solid tumours and treatment-resistant haematological malignancies, they are limited by a combination of factors, including poor in vivo persistence, immunosuppressive environmental factors and T cell exhaustion4.
Read about T cell therapy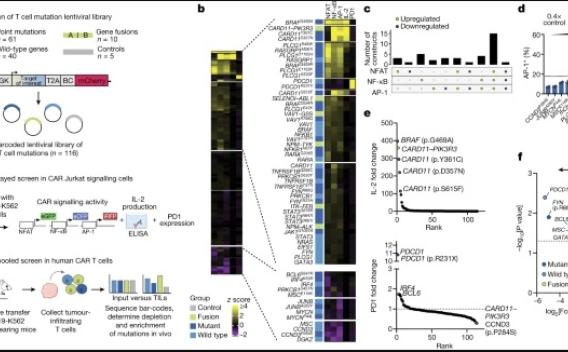
Systemic lupus erythematosus (SLE) is prototypical autoimmune disease driven by pathological T cell–B cell interactions1,2. Expansion of T follicular helper (TFH) and T peripheral helper (TPH) cells, two T cell populations that provide help to B cells, is a prominent feature of SLE.
Read more about T cell interaction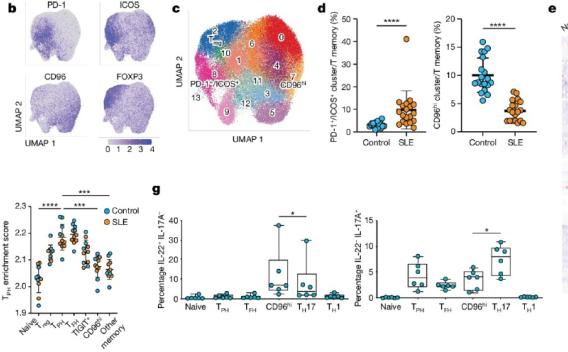
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine skin cancer with a ∼50% response rate to immune checkpoint blockade (ICB) therapy. In nonresponders, tumors show evidence of increased tumor proliferation, neuronal stem cell markers, and IL1. Responders have increased type I/II interferons and preexisting tissue resident (Trm) CD8 or Vδ1 γδ T cells that functionally converge with overlapping antigen-specific transcriptional programs and clonal expansion of public T-cell receptors.
Read more about how immunotherapy efficacy is predicted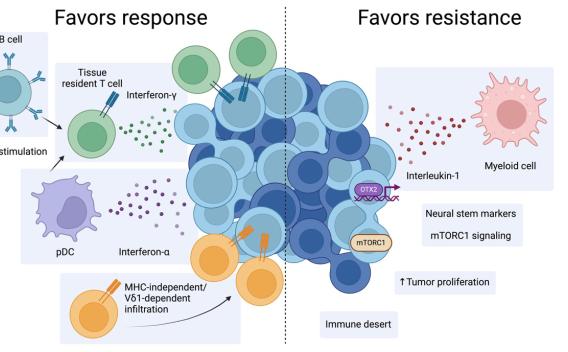
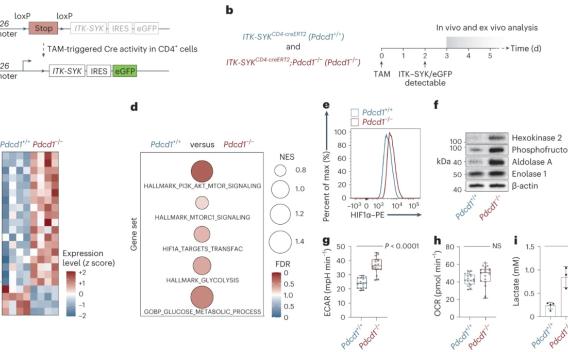
The PDCD1-encoded immune checkpoint receptor PD-1 is a key tumor suppressor in T cells that is recurrently inactivated in T cell non-Hodgkin lymphomas (T-NHLs). The highest frequencies of PDCD1 deletions are detected in advanced disease, predicting inferior prognosis.
T cell based immunotherapies have revolutionised the treatment of certain types of cancer. However these therapies — which involved taking someone’s own T cells and reprogramming them to kill cancer cells — have struggled to treat solid tumours, which put up multiple defences. To overcome these, a team has taken mutations found in cancer cells that help them thrive and put them into therapeutic T cells. Their results show these powered-up cells are more efficient at targeting solid tumours, but don’t turn cancerous themselves.
Research article: Garcia et al.
In this episode, Dr. Jaehyuk Choi from Northwestern University, explores Cutaneous T-Cell Lymphoma (CTCL), covering key diagnostic tools, emerging therapies, and the importance of a multidisciplinary team approach. He delves into effective symptom management, including the psychosocial impact of pruritus, and discusses the latest ongoing research. Additionally, Dr. Choi highlights disparities in diagnosis, treatment access, and patient outcomes. Tune in today to gain valuable insights on this complex diagnosis!
This episode is supported by Kyowa Kirin, Inc.
