We use cutting-edge approaches in cell biology, molecular biology, and genetics to study neurodegenerative disorders such as frontotemporal dementia (FTD), Lou Gehrig’s disease (amyotrophic lateral sclerosis; ALS), and Alzheimer’s disease.
Molecular mechanisms of anisosome formation in cells
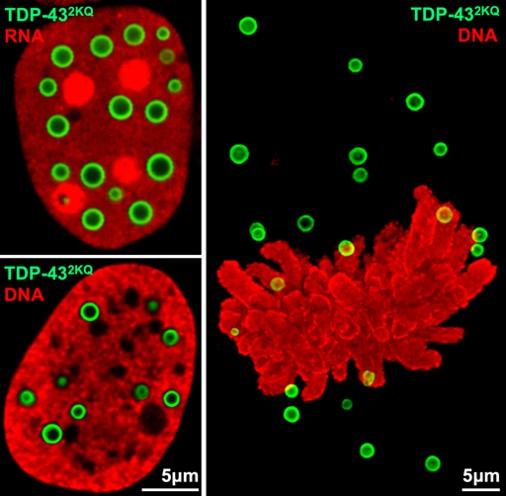 TDP-43 forms beautiful anisosomes (in green) in interphase and mitotic cells
TDP-43 forms beautiful anisosomes (in green) in interphase and mitotic cells A major focus of our research is TDP-43, a nuclear RNA-binding protein essential for neuronal health, which forms cytoplasmic aggregates during neurodegenerative disease. During Dr. Yu's postdoctoral research, he and his colleagues found that TDP-43 de-mixes into complex liquid-crystal droplets when it loses its ability to bind RNA. These droplets, which they named anisosomes, form in neurons in vivo when proteasome activity is inhibited, converting into aggregates when ATP levels fall.
Anisosomes have spherical shells of TDP-43 surrounding centers of HSP70 chaperones, whose activity maintains liquidity. They may contain other RNA-binding proteins with or without RNA in spinal muscular atrophy, a developmental motor neuron disease.
The Yu Lab will investigate how anisosomes form in living cells.
TDP-43 aggregation and neuronal RNA bodies
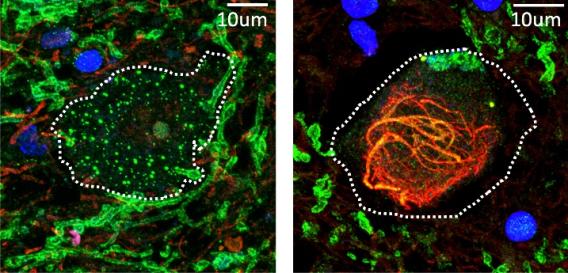 Left: RNA-processing bodies form droplets (green) in a healthy motor neuron. Right: RNA-processing bodies co-localize to TDP-43 aggregates (red) in an ALS motor neuron.
Left: RNA-processing bodies form droplets (green) in a healthy motor neuron. Right: RNA-processing bodies co-localize to TDP-43 aggregates (red) in an ALS motor neuron. TDP-43 forms cytoplasmic aggregates in the terminal stage of neurodegenerative diseases, such as ALS and frontotemporal dementia. I and colleagues found that when TDP-43 form aggregates in the cytoplasm, some cytoplasmic RNA-binding proteins were recruited to these aggregates, which was also observed in patient tissues.
We hypothesize that the aggregated TDP-43 may induce new stress on cells by interfering with the dynamics of RNA granules in the cytoplasm. The Yu Lab will determine how TDP-43 aggregation affects neuronal homeostasis by changing the dynamics of membraneless RNA granules.
New therapies for neurodegeneration
Many age-related neurodegenerative diseases are associated with intracellular protein aggregates:
- Tau in Alzheimer's disease and frontotemporal dementia
- TDP-43 in frontotemporal dementia and amytrophic lateral sclerosis
- Alpha-synuclein in Parkinson's disease.
We have developed a molecular glue that degrades aggregated proteins. We plan to further test whether molecular glues are effective against tau aggregates in the brain and to apply this approach against other types of protein aggregates.
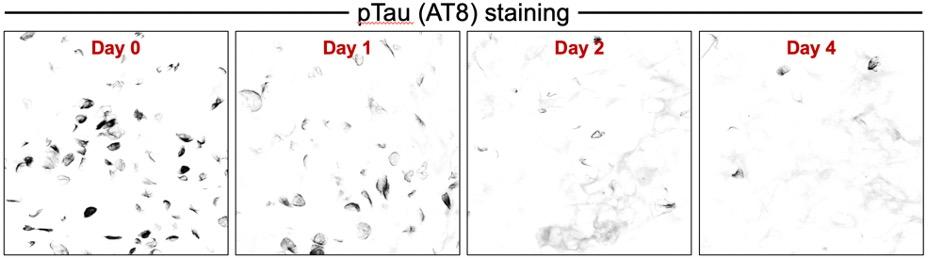 A molecular glue reduces tau aggregation (indicated by AT8 staining) in neuron-like cells
A molecular glue reduces tau aggregation (indicated by AT8 staining) in neuron-like cells