Are all metabolic changes during inflammation pathologic?
We study how metabolic adaptation promotes survival and tissue protection during sepsis.
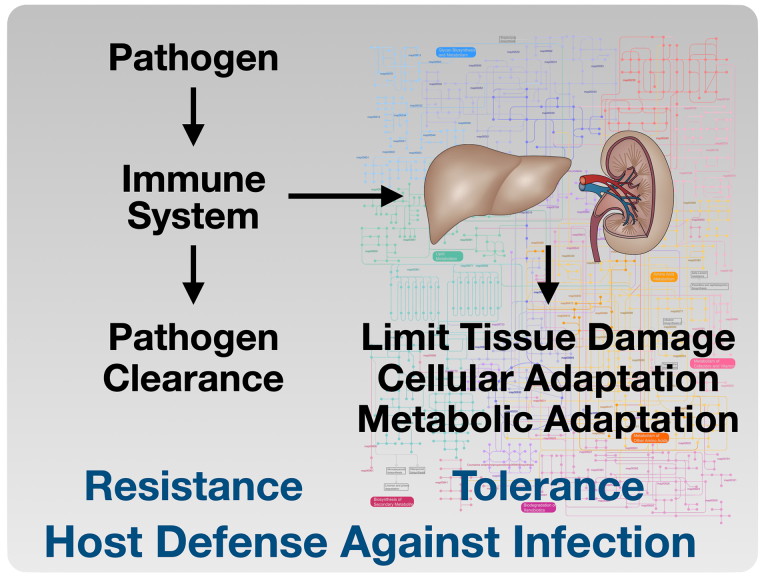
Sepsis is a significant cause of mortality among critically ill patients. Alterations in metabolism during sepsis are multifaceted and are incompletely understood as distinguishing the difference between protective and pathophysiologic responses is difficult.
Acute anorexia during infection is a highly conserved response, suggesting a protective role of anorexia in the host response to infection. The purpose of anorexia resulting from inflammation remains incompletely understood. The clinical implications of understanding how and why anorexia is a conserved sickness behavior are far-reaching. Optimal nutritional support in septic patients remains elusive, and controversy over the timing, nutritional composition, and route of administration persists.
Our prior work showed that caloric supplementation, specifically glucose, during the period of anorexia, increased mortality in mouse models of bacterial inflammation. Our approach to identify the mechanisms by which fasting metabolism is protective in bacterial inflammation includes the investigation of various fasting metabolic pathways using mouse models.
Current projects in the lab include:
- Determining potential mechanisms by which hepatic ketogenesis is dispensable in starvation adaptation.
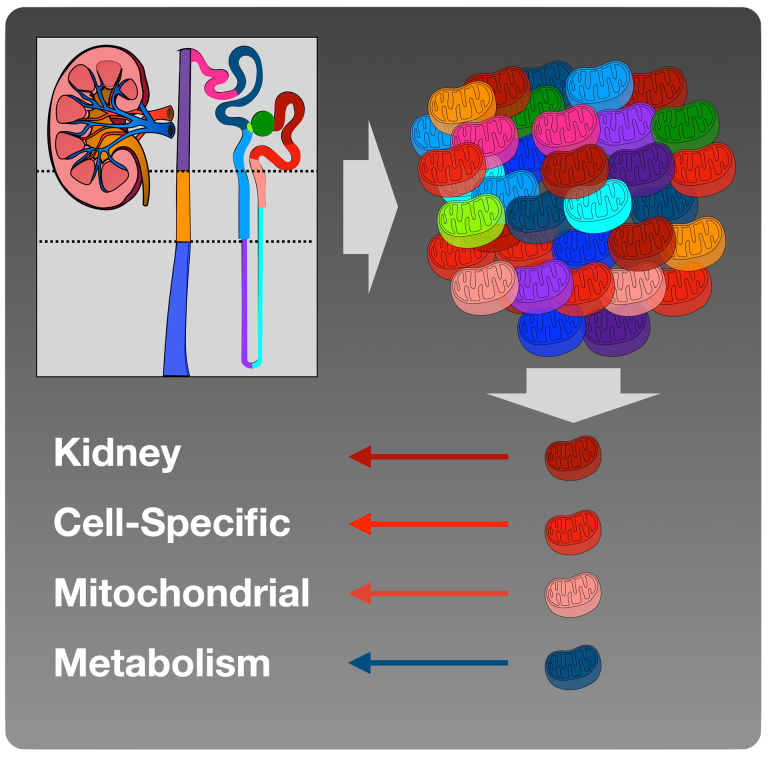
- Defining the role intra-renal ketogenesis and potential inter-cellular metabolite transfer.
- Characterizing kidney cell-specific mitochondrial metabolism.
- Understanding the role of lipid droplet formation in the kidney in response to fasting, inflammation, and ischemic injury