Cytoskeletal Control of Morphologic Signaling
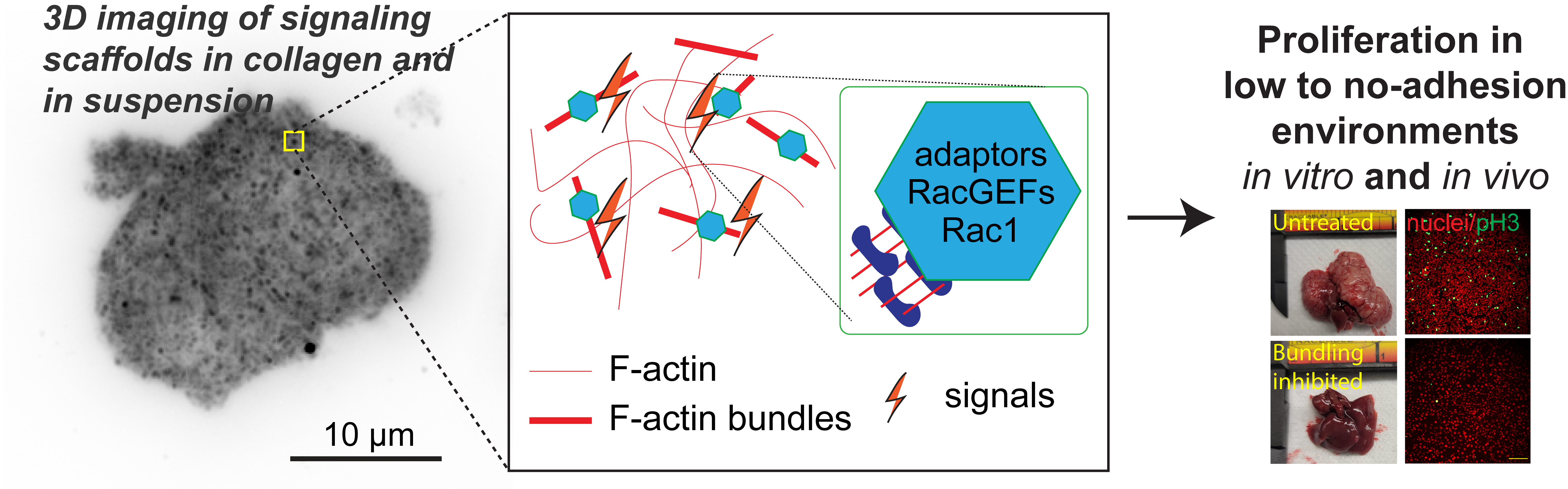
The cell cytoskeleton is composed of distinctive filamentous networks consisting of either actin, microtubules, intermediate filaments or septin polymers. Each network is configured into unique architectures through their respective binding partners and undergo dynamic remodeling in response to both cell-intrinsic and cell-extrinsic stimuli. We explore how distinct architectural networks govern cell signaling states. For example, we have contributed to showing how the branched actin structures inhibited NF2/merlin function and sustained proliferation of cancer cells under stress (Mohan et al., Dev Cell, 2019; Weiss et al., Under Revision) or how actin filament (F-actin) bundles sustained glycolysis in cancer cells under environments with low attachment (Park et al., Nature, 2020). Continuing this theme, we are currently characterizing a newly discovered signaling scaffold assembled at F-actin bundles that are implicated in cancer cell proliferation in vitro and in vivo in environments with limited to no extracellular matrix (Isogai et al., Under Revision). There are dozens of actin crosslinkers and actin binding proteins that could give rise to countless F-actin architectures with unique structural properties. The Isogai lab seeks to understand how cancer cells harness cytoskeletal filament assemblies to govern cell signaling states during development and in cancer.
Functional Causality in The Lamellipodial Actin Machinery
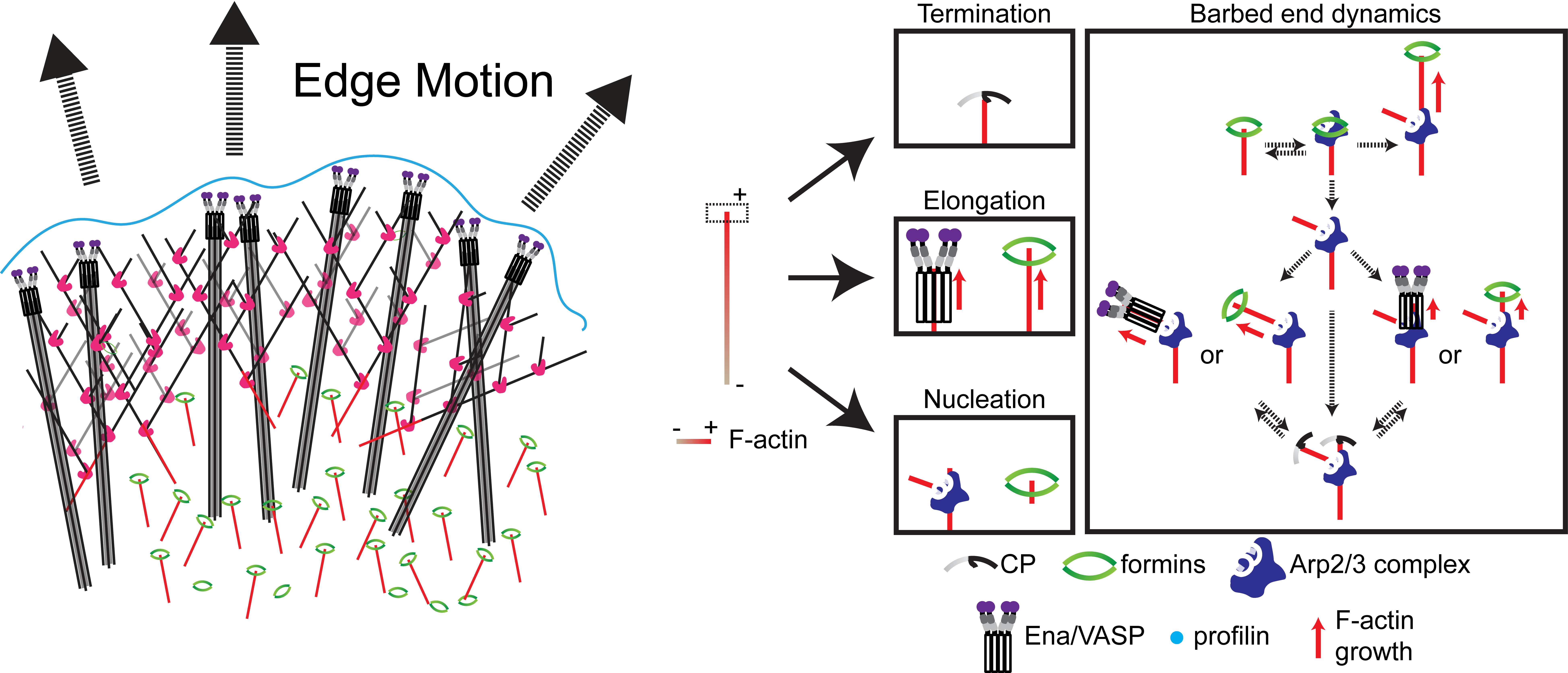
The study of complex molecular pathways is challenged by redundant and non-linear signaling circuits that are sensitive to instantaneous and long-term adaptation, consequently making traditional perturbation-based experiments difficult to interpret. To overcome this challenge, we have developed a perturbation-free statistical time-series analytical method to infer functional causal relationships among molecular components and used it to determine the hierarchy of actin regulators during cell membrane protrusions (Noh & Isogai et al., Cell Systems, 2022). This approach allowed us to distinguish correlative versus causal functional interactions of actin regulators during membrane protrusions, and we propose that the F-actin cytoskeleton at the cell leading edge is composed of at least two distinct F-actin structures, i.e., membrane-tethered bundles and branched actin, to drive cell membrane protrusions. Currently, we are using this framework to investigate the causal hierarchy of barbed-end regulation in migrating cells, and exploring morphological motifs that control cell state transitions in healthy and transformed cells.
Tissue morphogenesis in 3D environments
Cells dynamically change shape in response to extracellular environment through dynamic remodeling of the cell cytoskeleton. Cells form membrane protrusions or pseudopods in tissue-like environments that contribute to cell migration and invasion during developmental and metastatic processes, respectively, but their underlying mechanism is less understood than when cells are placed in planar plastic or glass tissue culture conditions. Using cutting-edge microscopy techniques and computer-assisted visualization and quantification tools, we seek to understand the contribution of actin-binding proteins in pseudopod formation.