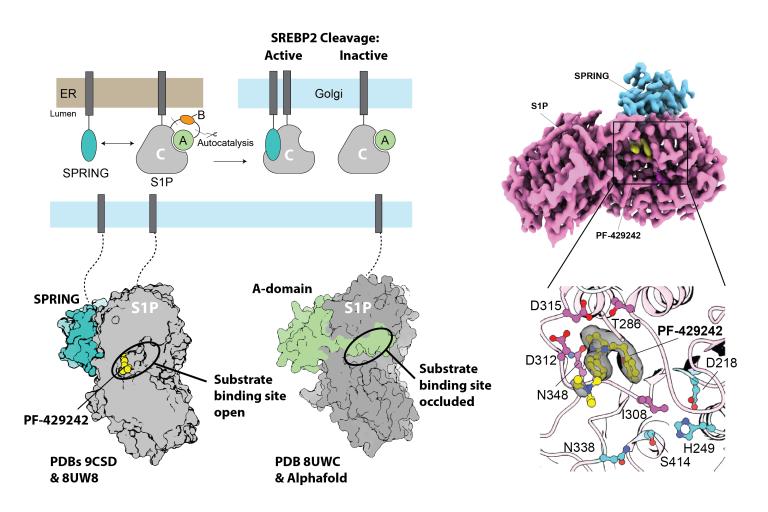
Maturation and activity of pro-protein convertases
Pro-protein convertases (PCSKs) are serine proteases in the secretory pathway that are required for numerous metabolic functions, including lipid metabolism. These proteases typically fold in the endoplasmic reticulum and undergo autocatalytic maturation. Our lab is interested in how chaperone proteins regulate PCSK maturation and activities.
Specific questions include:
1) How do chaperones control PCSK activity? So far, we have focused on how a chaperone called SPRING licenses activity by site-one protease, but we are interested in expanding these studies to the other PCSKs.
2) How is PCSK activity spatially controlled in cells? A common feature of PCSKs is that they are active is specific cellular compartments. How is this accomplished?
3) Can we develop new and improved versions of PCSK inhibitors by targeting chaperone-protease interactions?
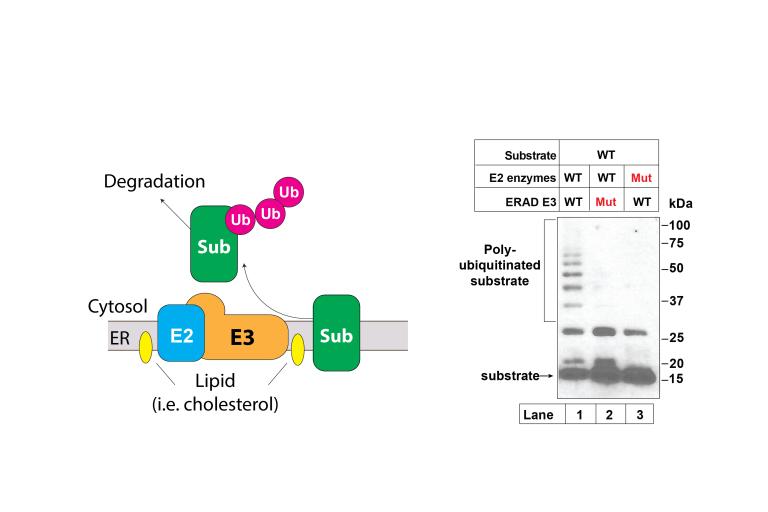
Understanding how membrane composition regulates protein degradation
Regulated protein degradation at the ER (ERAD) helps regulated lipid biosynthesis.
We are interested in understanding:
1) How do lipids in the membrane regulate ERAD?
2) How are ERAD substrates recognized?
3) Can we develop platforms to guide ERAD substrate identification?
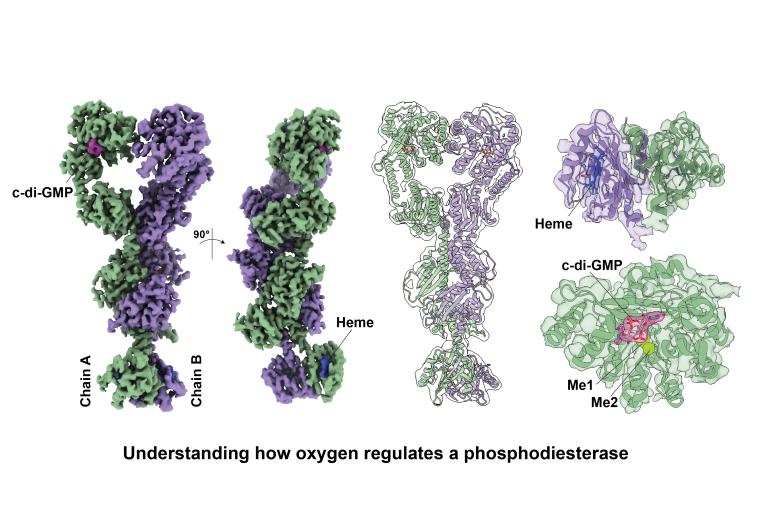
We like to have fun with others!
Our lab collaborates with other labs at UTSW wherever our expertise can be useful!
We have ongoing projects involving structure determination, protein-protein interactions, and regulated enzymology.