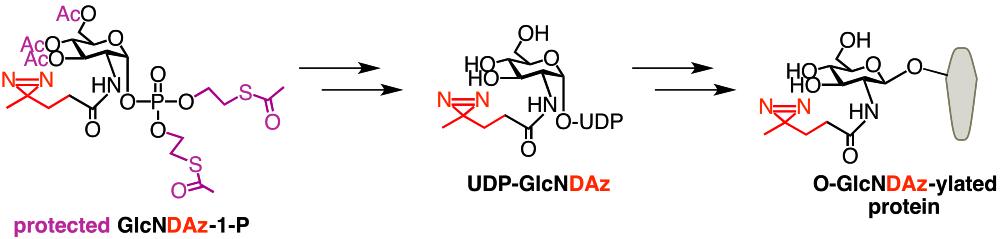
GlcNDAz is an analog of GlcNAc in which the diazirine photocrosslinking is appended at the N-acyl position. The enzymes of the human GlcNAc salvage pathway cannot efficiently metabolize GlcNDAz. Therefore, to achieve metabolic incorporation of GlcNDAz into cellular glycoconjugates, we synthesize the 1-phospho version of GlcNDAz, and also stably transfect cells with a mutant (F383G) of the UDP-GlcNAc pyrophosphorylase 1 (UAP1) that is capable of converting GlcNDAz-1-P to UDP-GlcNDAz. The GlcNDAz-1-P compound that we use includes protecting groups on the phosphate and all hydroxyl groups. These protecting groups make the compound less polar, allowing it to readily enter mammalian cells. Once inside the cell, intracellular esterases catalyze protecting group removal. Human OGT can transfer GlcNDAz from UDP-GlcNDAz to serines and threonines of substrate proteins, resulting in the production of the O-GlcNDAz modification in place of the naturally occurring O-GlcNAc modification. Cells containing O-GlcNDAz can be subjected to UV irradiation, leading to covalent crosslinking between O-GlcNAcylated proteins and nearby molecules. These crosslinked complexes can be analyzed by immunoblot and mass spectrometry. For example, we used O-GlcNDAz crosslinking to demonstrate the proximity between O-GlcNAcylated FG-repeat nucleoporins and karyopherins, such as transportin-1. We do not yet know whether GlcNDAz is incorporated into other types of glycoconjugates, such as N-linked glycans or GalNAc-type O-linked glycans.
Reagents available
- Lentivirus vector encoding UAP1(F383G)
- Protected GlcNDAz-1-P
- Cells lines stably expressing UAP1(F383G): Colo205, HeLa, T84, K562
- UDP-GlcNDAz (can be used for cell-free experiments)
O-GlcNDAz Crosslinking
The O-GlcNAc transferase (OGT) transfers GlcNDAz less efficiently than GlcNAc. We have also shown that a point mutation (C917A) to OGT enables a more efficient transfer of GlcNDAz. In addition, the O-GlcNAcase (OGA) that removes O-GlcNAc cannot remove O-GlcNDAz. We identified an OGA mutant (C215A) that can remove O-GlcNDAz. Achieving stable expression of OGT(C917A) and/or OGA(C215A) can be challenging because cells tightly regulate O-GlcNAc levels. Thus, we do not currently recommend routine use of OGT(C917A) and OGA(C215A) for crosslinking experiments, but the plasmids encoding them are available on request.
Reagents available
- Bacterial expression plasmid for OGT(C917A)
- Mammalian expression plasmid for OGT(C917A)
- Bacterial expression plasmid for OGA(C215A)
- Mammalian expression plasmid for OGA(C215A)
Related literature
The O-GlcNDAz crosslinking method
S-H Yu, M Boyce, AM Wands, MR Bond, CR Bertozzi, and JJ Kohler. Metabolic labeling enables selective photocrosslinking of O-GlcNAc-modified proteins to their binding partners. Proc. Natl. Acad. Sci. U. S. A. (2012) 109:4834-4839
A mutant of OGT with improved GlcNDAz transfer kinetics
AC Rodriguez, SH Yu, B Li, H Zegzouti, and JJ Kohler. Enhanced transfer of a photocrosslinking GlcNAc analog by an O-GlcNAc transferase mutant with converted substrate specificity. J. Biol. Chem. (2015) 290:22638-22648
A mutant of OGA that can remove O-GlcNDAz
AC Rodriguez, and JJ Kohler. Recognition of diazirine-modified O-GlcNAc by human O-GlcNAcase. MedChemComm (2014) 5:1227-1234
Funding
GlcNDAzOur research is funded by the NIH (R21DK112733) through the Common Fund Glycoscience Initiative.
Please visit the Common Fund Glycoscience Initiative website for other glycoscience tools under development.
Let's work together!
We are eager to have other research groups use our photocrosslinking sugar technology. To request reagents or discuss collaborative projects, please email Dr. Kohler.