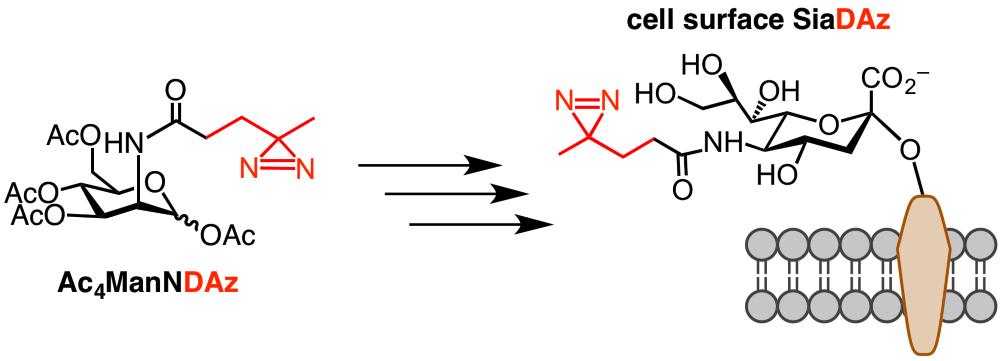
While we have previously prepared SiaDAz synthetically and added it to cultured cells, we find that it is more practical to use an analog of ManNAc, which is a committed precursor of Neu5Ac synthesis. The ManNAc analog (Ac4ManNDAz) contains the diazirine substituent and also has acetyl-protecting groups on all hydroxyl groups. These protecting groups reduce the polarity of the compound, allowing Ac4ManNDAz to readily enter cells where it is deprotected to ManNDAz. Enzymes in the sialic acid biosynthetic pathway convert ManNDAz to CMP-SiaDAz, and add SiaDAz to glycoproteins and glycolipids in place of other sialic acids, like Neu5Ac. Cells can then be subjected to UV (~350 nm) irradiation, resulting in covalent crosslinking between SiaDAz-containing glycoconjugates and nearby molecules. These covalent complexes can be analyzed by immunoblot and/or mass spectrometry. For example, we have used SiaDAz crosslinking to demonstrate that cholera toxin subunit B (CTB) can crosslink to both the glycolipid GM1 and fucosylated glycoproteins such as CEACAM5. One limitation of SiaDAz crosslinking technology is that the level of SiaDAz incorporation varies among different cell types, ranging from 0 – 75 % replacement of cell surface Neu5Ac. In general, we find that crosslinking can be achieved with incorporation levels of ~10 % or greater. We can help you measure SiaDAz incorporation levels in your cell line of interest.
Reagents available
- Ac4ManNDAz
Related literature
Use of Ac4ManNDAz to generate SiaDAz
Y Tanaka, and JJ Kohler. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J. Amer. Chem. Soc. (2008) 130:3278-3279
A protocol for preparing and using Ac4ManNDAz
2009 MR Bond, H Zhang, PD Vu, and JJ Kohler. Photocrosslinking of glycoconjugates using metabolically incorporated diazirine-containing sugars. Nat. Protoc. (2009) 4:1044-1063
Using Ac4ManNDAz to crosslink protein-glycolipid interactions
MR Bond, CM Whitman, and JJ Kohler. Metabolically incorporated photocrosslinking sialic acid covalently captures a ganglioside-protein complex. Mol. Biosys. (2010) 6:1796-1799
Application of Ac4ManNDAz to discover novel CTB receptors
AM Wands, A Fujita, JE McCombs, J Cervin, B Dedic, AC Rodriguez, N Nischan, MR Bond, M Mettlen, DC Trudgian, A Lemoff, M Quiding-Järbrink, B Gustavsson, C Steentoft, H Clausen, H Mirzaei, S Teneberg, U Yrlid, and JJ Kohler. Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife (2015) 4:e09545
Incorporation of SiaDAz depends on cell type
2015 ND Pham, CS Fermaintt, AC Rodriguez, JE McCombs, N Nischan, and JJ Kohler. Cellular metabolism of unnatural sialic acid precursors. Glycoconj. J. (2015) 32:515-529
Funding
SiaDAz
Our research is funded by the NIH (R21DK112733) through the Common Fund Glycoscience Initiative.
Please visit the Common Fund Glycoscience Initiative website for other glycoscience tools under development.
Let's work together!
We are eager to have other research groups use our photocrosslinking sugar technology. To request reagents or discuss collaborative projects, please email Dr. Kohler.