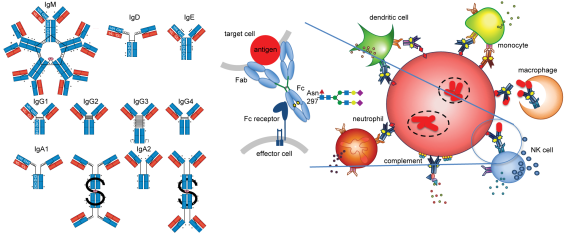
For decades, the field of tuberculosis (TB) immunology has focused on T cell mediated protection, yet Mycobacterium tuberculosis (Mtb) still impacts one in four individuals worldwide today. While T cells are clearly important, gaps in this paradigm remain, and a role for the other arm of the immune system – humoral immunity – is less clear. Antibodies connect host and microbe by the combination of antigen recognition by the Fab domain and linking to the host by the Fc domain. Despite the nomenclature, the Fc is dynamic and heterogenous: changes in isotypes, subclasses, and post translational modifications such as glycosylation modulate Fc affinity for Fc receptors, impacting diverse immune effector functions. In the rheumatologic and oncologic fields, polyclonal antibody responses are promising immune correlates for biomarker development and monoclonal antibody therapeutic Fc engineering have led to clinically significant benefits. In infections, the impacts of the Fc are complex, mediating protection as well as enhancing disease.
A systems serology approach was used to show divergent humoral profiles in two classical human states of TB: latent infection (LTB), with no microbiological burden or signs and symptoms of disease yet a T cell signature of infection, and active TB disease (ATB), with the presence of detectable Mtb, signs and symptoms of disease, as well as a T cell signature of infection. These differences in antibody landscapes are highlighted by Fc glycosylation and reflected by differential Mtb burden and antimicrobial processes in an in vitro macrophage model of infection. These findings lead to exciting new questions:
- What is the relevant antigenic repertoire?
- Which Fc glycan features are important and why?
- How do antibodies direct intracellular bacterial fate within and between immune cells?
- How do additional human immune states inform on the relevant responses to Mtb?
These studies together provide insight into the collective immune pressures that shape how infection progresses to disease by identifying key correlates in humans to be further investigated in models in vitro and in vivo. Beyond TB, the study of antibodies in infectious diseases helps construct a more in-depth framework to understand how the landscape of humoral immunity mediates the interface of host microbial interactions, with outcomes that broaden the potential for antibody-based diagnostics, therapeutics, and vaccine development.