Vascular disrupting agents promise effective tumor control by targeting the unique fragile blood vessels associated with tumor angiogenesis and growth. Lead agents have shown promise in clinical trials, but there is a clear need for further specificity, greater efficacy, and reduced side effects. Many new diverse molecular structures are being considered, but following initial testing in culture, evaluation in pre-clinical small animals models is vital prior to conceivable human clinical trials.
We have applied various imaging stratagems to assess changes in tumor vasculature in response to VDAs: notably MRI (1H and 19F), near infrared spectroscopy, dynamic bioluminescence, dynamic fluorescence, and ultrasound. These studies are currently under active development in collaboration with Kevin Pinney, Ph.D. and Mary Lynn Trawick, Ph.D. of Baylor University.
We have also explored other vascular targeting agents, which exploit unique characteristics of tumors; these agents may be used to detect tumors or as potential therapeutics but generally do not cause acute vascular disruption. These studies were pursued in collaboration with Sally Ward-Ober, Ph.D., Alan Schroit, Ph.D., and Rolf Brekken, Ph.D., Raimund Ober, Ph.D. of UT Dallas; and were initiated with the late Phil Thorpe, Ph.D.
References
VDAs
Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment L. Liu, R. Schuetze, J. L. Gerberich, R. Lopez, S. O. Odutola, R. P. Tanpure, A. K. Charlton-Sevcik, J. K. Tidmore, E A.-S. Taylor, P. Kapur, H. Hammers, M L. Trawick, K. G. Pinney, and R. P. Mason, Cancers 14(17: 4208 2022
Imaging Guided Evaluation of the Novel Small-Molecule Benzosuberene Tubulin-Binding Agent KGP265, as a Potential Therapeutic Agent for Cancer Treatment, Y. Guo, H. Wang, J.L. Gerberich, S.O. Odutola, A.K. Charlton-Sevcik, M. Li, R.P. Tanpure, J.K. Tidmore, Y. Wang, M.L. Trawick, K.P. Pinney, R.P. Mason, and L. Liu, Cancers., 13(19), 4769, 2021.
Non-invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors. L. Liu, D. O’Kelly, R. Schuetze, G. Carlson, H. Zhou, M. L. Trawick, K. G. Pinney and R. P. Mason Molecules, 2021, 26, 2551, DOI: 10.3390/molecules26092551 PMCID: PMC8125421
Bioreductively Activatable Prodrug Conjugates of Combretastatin A-1 and Combretastatin A-4 as Anticancer Agents Targeted Towards Tumor-Associated Hypoxia, B. A. Winn, L. Devkota, B. Kuch, M. T. MacDonough, T. E. Strecker, Y. Wang, Z. Shi, J. L. Gerberich, D. Mondal, A. J. Ramirez, E. Hamel, D. J. Chaplin, P. Davis, R. P. Mason, M. L. Trawick, K. G. Pinney, J. Nat. Prod., 83, 937-954, 2020.
Noninvasive Anatomical and Functional Imaging of Orthotopic Glioblastoma Development and Therapy using Multispectral Optoacoustic Tomography, G. Balasundaram, L. Ding, L. Xiuting, A. Attia, X. Luis, D. Ben, C. Jun, H. Ho, P. Chandrasekharan, H. C. Tay, H. Q. Lim, C. Bing Ong, R. P. Mason, D. Razansky, M. Olivo, Trans. Oncol., 11, 1251-1258 (2018).
Evaluation of tumor ischemia in response to an indole-based vascular disrupting agent using BLI and (19)F MRI. Zhou H, Hallac RR, Lopez R, Denney R, MacDonough MT, Li L, Liu L, Graves EE, Trawick ML, Pinney KG, Mason RP Am J Nucl Med Mol Imaging 2015 5 2 143-53.
The vascular disrupting activity of OXi8006 in endothelial cells and its phosphate prodrug OXi8007 in breast tumor xenografts. Strecker TE, Odutola SO, Lopez R, Cooper MS, Tidmore JK, Charlton-Sevcik AK, Li L, MacDonough MT, Hadimani MB, Ghatak A, Liu L, Chaplin DJ, Mason RP, Pinney KG, Trawick ML Cancer Lett. 2015 Sep.
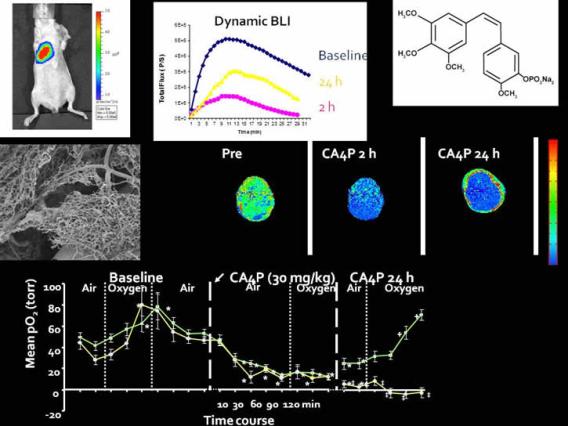
Dynamic bioluminescence and fluorescence imaging of the effects of the antivascular agent Combretastatin-A4P (CA4P) on brain tumor xenografts. Liu L, Mason RP, Gimi B Cancer Lett. 2015 Jan 28;356(2 Pt B):462-9.
Dynamic contrast enhanced fluorescent molecular imaging of vascular disruption induced by combretastatin-A4P in tumor xenografts. Liu L, Su X, Mason RP J Biomed Nanotechnol 2014 Aug 10 8 1545-51.
A perspective on vascular disrupting agents that interact with tubulin: preclinical tumor imaging and biological assessment. Mason, R.P., Zhao D., Liu L., Trawick M.L., Pinney K.G. Integr Biol (Camb) 2011 Apr 3 4 375-87.
Synthesis of a 2-Aryl-3-Aroyl-Indole Salt (OXi8007) Resembling Combretastatin A-4 with Application as a Vascular Disrupting Agent. M. B. Hadimani, M. T. MacDonough, A. Ghatak, T. E. Strecker, R. Lopez, M. Sriram, B. L. Nguyen, R. J. Kessler, A. R. Shirali, L. Liu, C. M. Garner, G. Pettit, R. E. Hamel, D. J. Chaplin, R. P. Mason, M. L. Trawick, K. G. Pinney, J. Nat. Prod., 76(9):1668-78, DOI: 10.1021/np400374w 2013
Comparison of optical and power Doppler ultrasound imaging for non-invasive evaluation of arsenic trioxide as a vascular disrupting agent in tumors. Alhasan M.K., Liu L., Lewis M.A., Magnusson J., Mason, R.P. PLoS ONE 2012 7 9 e46106.
In vivo near-infrared spectroscopy and magnetic resonance imaging monitoring of tumor response to combretastatin A-4-phosphate correlated with therapeutic outcome. Zhao D., Chang C.H., Kim J.G., Liu H, Mason, R.P. Int. J. Radiat. Oncol. Biol. Phys. 2011 Jun 80 2 574-81.
Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging and confirmed by MRI. Zhao D., Richer E., Antich P.P., Mason, R.P. FASEB J. 2008 Jul 22 7 2445-51.
Tumor physiologic response to combretastatin A4 phosphate assessed by MRI. Zhao D., Jiang L., Hahn E.W., Mason, R.P. Int. J. Radiat. Oncol. Biol. Phys. 2005 Jul 62 3 872-80.
Oxygenation in a human tumor xenograft: manipulation through respiratory challenge and antibody-directed infarction. Mason, R.P., Constantinescu A., Ran S., Thorpe P.E. Adv. Exp. Med. Biol. 2003 530 197-204.
“Structural Interrogation of Benzosuberene-Based Inhibitors of Tubulin Polymerization” C. A. Herdman, L. Devkota, C.-M. Lin, H. Niu, T. E. Strecker, R. Lopez, L. Liu, C. S. George, R. P. Tanpure, E. Hamel, D. J. Chaplin, R. P. Mason, M. L. Trawick, and K. G. Pinney, Bioorg. Med. Chem., 23(24), 7497–7520 (2015) doi: 10.1016/j.bmc.2015.10.012
“Design, Synthesis, and Biological Evaluation of Water-Soluble Amino Acid Prodrug Conjugates Derived from Combretastatin, Dihydronaphthalene, and Benzosuberene-Based Parent Vascular Disrupting Agents" ”, L. Devkota, C.-M. Lin, T. E. Strecker, Y. Wang, J. K. Tidmore, Z. Che, R. Guddneppanavar, C. J. Jelinek, R. Lopez, L. Liu, E. Hamel, R. P. Mason, D. J. Chaplin, M. L. Trawick, and K. G. Pinney, Bioorg. Med. Chem. 24: 938–956 (2016), doi: 10.1016/j.bmc.2016.01.007
Synthesis and Biological Evaluation of Benzocyclooctene-based and Indene-based Anticancer Agents that Function as Inhibitors of Tubulin Polymerization C. A. Herdman, T. E. Strecker, R. P. Tanpure, Z. Chen, A. Winters, J. Gerberich, L. Liu, E. Hamel, R. P. Mason, D. J. Chaplin, M. L. Trawick, and K. G. Pinney, Med. Chem. Commun., 7, 2418 2427, (2016) DOI: 10.1039/C6MD00459H
Synthesis of dihydronaphthalene analogues inspired by combretastatin A-4 and their biological evaluation as anticancer agents. C. J. Maguire, Z. Chen, V. P. Mocharla, M. Sriram, T. E. Strecker, E. Hamel, H. Zhou, R. Lopez, R. P. Mason, D. J. Chaplin, M. L. Trawick, K. G. Pinney, MedChemComm 9 (10):1649-1662; OCT 1 2018
VTAs
Phosphatidylserine-targeted molecular imaging of tumor vasculature by magnetic resonance imaging. Zhou H, Stafford JH, Hallac RR, Zhang L, Huang G, Mason RP, Gao J, Thorpe PE, Zhao D J Biomed Nanotechnol 2014 May 10 5 846-55.
Vascular imaging of solid tumors in rats with a radioactive arsenic-labeled antibody that binds exposed phosphatidylserine. Jennewein M., Lewis M.A., Zhao D., Tsyganov E., Slavine N., He J., Watkins L., Kodibagkar V.D., O'Kelly S., Kulkarni P., Antich P.P., Hermanne A., Rösch F., Mason, R.P., Thorpe P.E. Clin. Cancer Res. 2008 Mar 14 5 1377-85.