Our lab focuses on the function of the proteins that control plasma membrane function. We have on-going projects investigating ARH/LDLR endocytosis and caveolae signal transduction.
ARH
We study the mechanisms by which the protein ARH supports LDLR-dependent LDL uptake, but is dispensable for LDLR-dependent VLDL uptake.
LDLR
Our research has shown how the LDLR responds to low pH and low calcium concentrations to release lipoprotein in endocytic vesicles.
Springs
By modifying amino acid residues of the helical protein ankyrin (ANK), we can alter its shape. We are now developing engineered ANK-repeat protein springs for making nanodevices.
Cavin-3
Cavin-3 is a tumor suppressor protein whose loss leads to cachexia (wasting), a frequent cause of death in cancer.
Ankyrin (ANK) repeats stack side-by-side to form helical concatemers that resemble nanometer-sized metal springs. Using the native 24-repeat stack from human ankyrin, we showed that helical shaped ANK-repeat proteins not only look like springs, they also function like springs.
We have identified residue modification that control the shape of the helices formed by the repeats and are now developing engineered ANK-repeat protein springs for the purpose of making nanodevices.
Publications
- Davis L, Abdi K, Machius M, Brautigam C, Tomchick DR, Bennett V, Michaely P. Localization and structure of the ankyrin-binding site on beta2-spectrin. J Biol Chem. 2009 284:6982-7. PMID: 19098307.
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behavior of ankyrin repeats. Nature. 2006 440:246-9. PMID: 16415852.
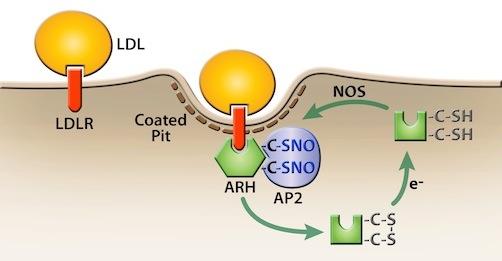 Role of nitrosylation in ARH-dependent LDL uptake
Role of nitrosylation in ARH-dependent LDL uptake The protein autosomal recessive hypercholesterolemia (ARH) supports LDLR-dependent LDL uptake, but is dispensable for LDLR-dependent VLDL uptake.
Our work has examined the molecular mechanisms responsible for this lipoprotein specificity. We have shown that the LDLR has two internalization sequences:
- The FDNPVY sequence is a constitutive uptake sequence that supports internalization irrespective of whether or not lipoprotein is bound.
- The HIC sequence is a VLDL-induced uptake sequence that only supports internalization when VLDL is bound. ARH binds to the FDNPVY sequence and supports the constitutive uptake pathway, which is necessary for LDL uptake.
In recent work we showed that ARH activity requires nitrosylation, a post-translational modification of cysteine sulfhydryls by nitric oxide. Nitrosylation activates the ability of ARH to bind to components of clathrin coated pits and is necessary for ARH to drive LDLRs to this endocytic structure.
One consequence of ARH involvement is that the distribution of receptors between the cell surface and intracellular compartments shifts toward the latter. Loss of ARH function increases the number of surface receptors at steady state and this increase can improve VLDL uptake.
Thus, regulation of nitric oxide levels allows cells that use ARH to control whether the LDLR internalizes both LDL and VLDL or functions as a super-potent VLDL receptor.
Publications
- Zhao Z, Pompey S, Dong H, Weng J, Garuti R, Michaely P. S-nitrosylation of ARH is required for LDL uptake by the LDL receptor. J Lipid Res. 2013 54:1550-9. PMID: 23564733.
- Michaely P, Zhao Z, Li WP, Garuti R, Huang L, Hobbs HH, Cohen JC. Identification of a VLDL-induced, FDNPVY-independent internalization mechanism for the LDLR. EMBO J 2007 26: 3273-82. PMID: 17581630.
- Jones C, Garuti R, Michaely P, Li WP, Maeda N, Cohen JC, Herz J, Hobbs HH. Disruption of LDL but not VLDL clearance in autosomal recessive hypercholesterolemia. J Clin Invest. 2007 117: 165-174. PMID: 17200716.
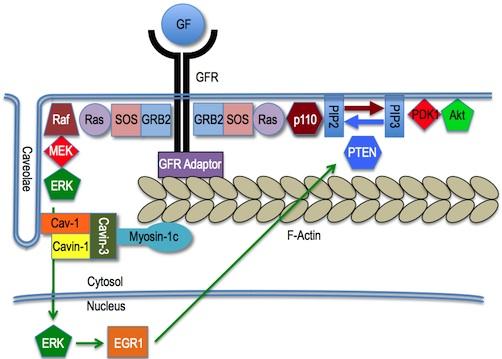 Role of cavin-3 in crosstalk between ERK and Akt
Role of cavin-3 in crosstalk between ERK and Akt Cavin-3 (PRKCDBP, hSRBC) is a tumor suppressor protein that is commonly absent in epithelial cancers (carcinomas), particularly late in disease progression. Frequency of loss in primary tumors ranges from 50 percent to 80 percent, depending on cancer type.
Our work has shown that cavin-3 dictates the balance between ERK and Akt signaling: increases in cavin-3 function increase ERK activity over Akt, while loss of cavin-3 function increases Akt activity over ERK.
The cellular consequences of cavin-3 loss include Warburg metabolism (aerobic glycolysis), rapid cell proliferation, and resistance to cell death (apoptosis).
Whole-body cavin-3 knockout in mice increases Akt signaling and glycolytic metabolism in the tissues, among other effects. Animals die prematurely of cachexia, a wasting syndrome characterized by severe lipodystrophy and muscle loss.
A role for cavin-3 as a bulwark against cachexia is consistent with the observation that cancers with high incidence of cancer-associated cachexia (CAC) are the cancers that most commonly lack cavin-3 expression.
We are currently investigating how loss of cavin-3 causes cachexia. CAC is the immediate cause of death in 20 percent of all cancer patients, and at least half of all cancer patients experience some level of cachexia.
Publications
- Hernandez VJ, Weng J, Ly P, Pompey S, Dong H, Mishra L, Schwarz M, Anderson RG, Michaely P. Cavin-3 dictates the balance between ERK and Akt signaling. Elife. 2013 2:e00905. PMID: 24069528.
The low-density lipoprotein receptor (LDLR) internalizes lipoproteins (LDL, VLDL) as part of a cycle between the cell surface and endosomes.
The cycle starts at the cell surface where the LDLR binds to lipoprotein.These LDLR-lipoprotein complexes are internalized through clathrin-coated pits into small endocytic vesicles.
These vesicles rapidly acidify and lose calcium, which together drives release of lipoprotein from the LDLR. The released lipoprotein remains in the endocytic vesicles, while the LDLR is trafficked back to the cell surface for further rounds of uptake.
Lipoprotein that is released in endocytic vesicles is trafficked to lysosomes where their cholesterol and fatty acid contents are liberated for use by the cell.
Work performed in our lab has shown how the LDLR responds to low pH and low calcium concentrations to release lipoprotein in endocytic vesicles.
Publications
- Pompey SN, Michaely P, Luby-Phelps K. Quantitative Fluorescence Co-localization of Lipoproteins with the LDLR. Methods Mol Biol. 2013 1008:439-53. PMID: 23729262.
- Pompey S, Zhao Z, Phelps K, Michaely P. Quantitative fluorescence imaging reveals point of release for lipoproteins during LDLR-dependent uptake. J Lipid Res. 2013 54:744-53. PMID: 23296879.
- Zhao Z, Michaely P. Role of an intramolecular contact on lipoprotein uptake by the LDL receptor. Biochim Biophys Acta. 2011 1811:397-408 PMID: 21511053.
- Zhao Z, Michaely P. The role of calcium in lipoprotein release by the low-density lipoprotein receptor. Biochemistry. 2009 48:7313-24. PMID: 19583244.
- Zhao Z, Michaely P. The EGF-homology domain of the LDL receptor drives lipoprotein release through an allosteric mechanism involving H190, H562 and H586. J. Biol. Chem. 2008 283: 26528-37. PMID: 18677035.