About the ALF Study Group
The Acute Liver Failure Study Group (ALFSG) was a clinical research network funded by the National Institutes of Health from 1997 to 2023. Its mission was to collect prospective data and biological samples on acute liver failure (ALF), a rare but life-threatening condition.
The ALFSG comprised 33 sites in the U.S. and Canada. Over 160 original research articles have been published by ALFSG investigators and collaborators worldwide.
The group enrolled 3,364 patients with this rare condition in the registry and has maintained a biorepository of over 100,000 unique patient bio-samples, supporting a wide range of clinical trials and studies.
Although the registry stopped enrolling new patients in August 2019, the group continues to support new research protocols and requests for data or bio-samples, including serum, plasma, urine, tissue, and DNA.
Scope and Enrollment
Over 23 years, ALFSG–based at UT Southwestern Medical Center in Dallas–enrolled more than 2,631 patients with ALF and 857 with acute liver injury (ALI) across North America.
- ALF is defined as a severe form of acute liver injury characterized by rapid-onset over days and weeks liver injury without underlying cirrhosis, marked by abnormal coagulation with prolongation of the prothrombin time (INR ≥ 1.5) and altered mental status.
- ALI involves severe liver injury with an INR ≥ 2.0 but without encephalopathy (altered mental functioning, drowsiness, or coma).
ALF Causes and Outcomes
A unique feature of ALF and ALI is that there are a variety of causes, all of which share similar clinical features (coagulopathy, encephalopathy, susceptibility to infection, and bleeding) regardless of cause.
The most common cause (etiology) of ALF in North America is acetaminophen liver toxicity, in most instances the result of overdosing at a single time point (suicide attempt) or over time when seeking pain relief (unintentional overdose). Acetaminophen a very common pain reliever found in innumerable over-the-counter medications (Tylenol®, Nyquil®, TylenolPM®) and prescription opioid combination medications (Vicodin®, Percocet®).
Since APAP is a dose-related toxin, most severe liver injuries are the result of dosing above the recommended package labeling. However, there may be some instances where acetaminophen taken within the package instructions can cause severe acute liver injury and even fatalities. Other causes of ALF include toxicity secondary to prescription drugs, herbal and dietary supplements, and hepatitis A and B. In a small percentage of cases (less than 10%) the cause cannot be delineated (indeterminate).
The most frequent causes include:
- Acetaminophen (APAP) overdose (intentional or unintentional),
- Drug-induced liver injury,
- Autoimmune hepatitis,
- Hepatitis B.
Less common causes include acute fatty liver of pregnancy, ischemia/shock, Wilson disease, heat stroke, and metastatic cancer.
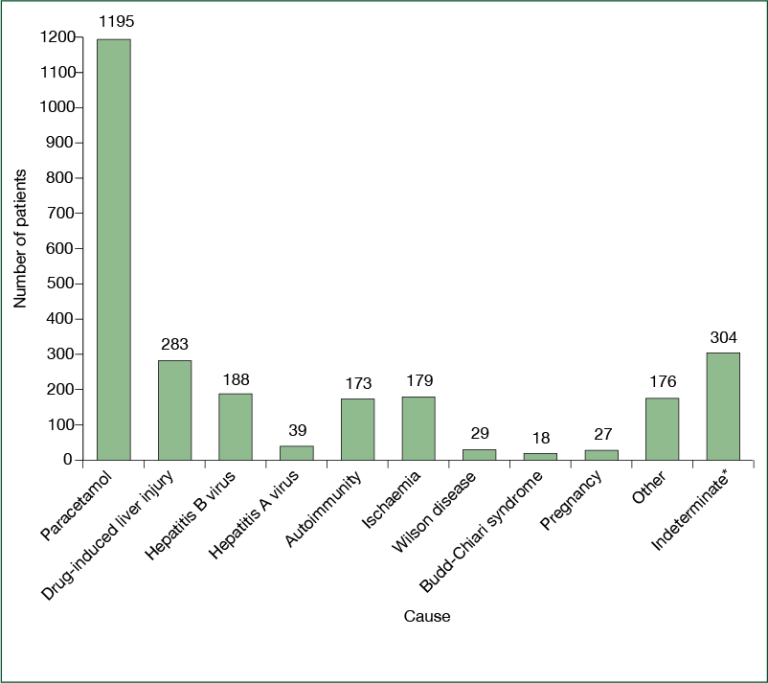 Causes of acute liver failure, as recorded by the site principal investigator in the US Adult Acute Liver Failure Study Group Registry
Causes of acute liver failure, as recorded by the site principal investigator in the US Adult Acute Liver Failure Study Group Registry The most common cause (etiology) of ALF in North America is acetaminophen liver toxicity, in most instances the result of overdosing at a single time point (suicide attempt) or over time when seeking pain relief (unintentional overdose). Acetaminophen a very common pain reliever found in innumerable over-the-counter medications (Tylenol®, Nyquil®, TylenolPM®) and prescription opioid combination medications (Vicodin®, Percocet®).
Since APAP is a dose-related toxin, most severe liver injuries are the result of dosing above the recommended package labeling. However, there may be some instances where acetaminophen taken within the package instructions can cause severe acute liver injury and even fatalities. Other causes of ALF include toxicity secondary to prescription drugs, herbal and dietary supplements, and hepatitis A and B. In a small percentage of cases (less than 10%) the cause cannot be delineated (indeterminate).
Each year, ALF affects approximately 2,000 people in the U.S., while ALI affects 2,000 to 4,000 individuals. Among ALF patients:
- 29% die,
- 22% undergo liver transplantation,
- 51% survive and typically recover fully.
Key Clinical Trials
ALFSG conducted several important trials, including:
- NAC Trial - N-acetylcysteine (NAC) for non-acetaminophen ALF
- STOP-ALF - Safety and Tolerability of Ornithine Phenylacetate for the management of hepatic encephalopathy in the setting of ALF
- ROTEM - Rotational Thromboelastography (ROTEM) was explored to determine whether abnormal hemostasis contributes to bleeding events, illness severity, or outcome.,
- MBT - C13-labeled methacetin breath test to evaluate liver function and predict outcomes in transplant candidates.
Mobile Tools for Clinicians
The Acute Liver Failure Study Group (ALFSG) has developed two mobile applications to support clinicians managing acute liver failure (ALF) cases:
A web version of the checklist is available for users who do not have access to an iPhone® or iPad®. You can access it here: Acute Liver Failure Checklist.
Availability
Both apps are available for download in the Apple App Store. They were developed to assist healthcare professionals in making timely, informed decisions when treating critically ill ALF patients.
Current Status and Access to Data
The ALFSG registry officially closed on August 31, 2019, and the database is now locked. However, researchers can still request access to data and bio-samples for approved studies.
As of two years post-closure, management of the registry and samples was transferred to the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases).
Researchers interested in accessing these resources must now contact the NIDDK repository directly. To contact NIDDK repository to set up your unique account, Email: niddk-crsupport@niddk.nih.gov. This will be your contact point for requesting and accessing data and samples. Several steps are required including submission of a research proposal and specification of types of samples/data you require. While Dr. William M. Lee and Dr. Jody Rule continue to review proposals informally, they no longer have authority to release samples or grant permissions. Feel free to contact them for additional information: Email willian.lee@utsouthwestern.edu or Email jody.rule@utsouthwestern.edu
Consent and Confidentiality
Because ALF patients often experience altered mental status, consent for participation must be provided by a next of kin or someone with medical power of attorney. ALI patients, however, can provide consent directly.
All collected data is de-identified, and the ALFSG holds a Certificate of Confidentiality from the National Institute of Mental Health.

